Evaluation of the Efficacy of Neoadjuvant Combined Therapy in Patients with Locally Advanced Head and Neck Squamous Cell Carcinoma
DOI:
https://doi.org/10.71321/dwr8tx96Keywords:
Head and neck squamous cell carcinoma, Neoadjuvant therapy, Targeted therapy, ImmunotherapyAbstract
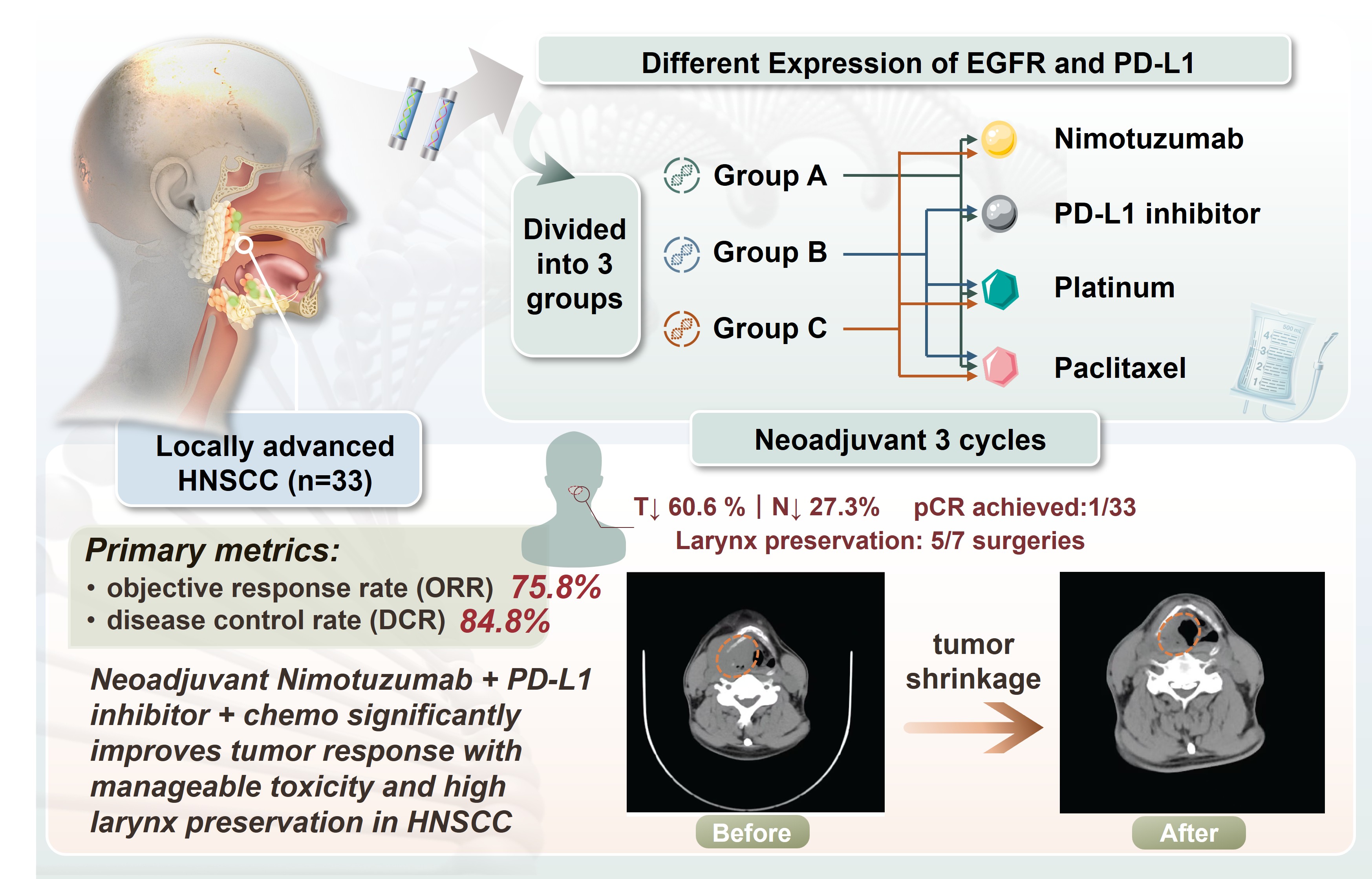
Objective: Our study aimed to assess the effectiveness and safety of neoadjuvant therapy involving the immune checkpoint inhibitor Nimotuzumab and programmed cell death ligand 1 (PD-L1) inhibitors, along with platinum and paclitaxel chemotherapy in individuals diagnosed with the head and neck squamous cell carcinoma (HNSCC).
Method: Over the course of 2024 and 2025, a cohort of 33 patients with locally advanced HNSCC admitted to our institution was included in the investigation. Stratification into three groups was based on the expression levels of epidermal growth factor receptor (EGFR) and PD-L1 in tumour tissues. The patients were treated with Nimotuzumab and PD-L1 inhibitors along with platinum and paclitaxel chemotherapy for three cycles. After three cycles of neoadjuvant therapy, some patients were given surgical treatment. The primary metrics for evaluating treatment success were the objective response rate (ORR) and the disease control rate (DCR). Secondary endpoints included pathological complete response rate, laryngeal preservation rate, and incidence of chemotherapy-related toxicities.
Results: The study results revealed promising tumour response among the 33 patients, with an objective response rate of 75.8%. One patient achieved a complete radiographic response. Twenty patients achieved radiologic response, with T-stage downstaging observed in 60.6% and N-stage downstaging in 27.3% of these cases. Seven patients underwent surgery following neoadjuvant therapy, with five of these patients successfully undergoing larynx-preserving hypopharyngeal cancer radical resections. Regarding adverse events, 21.2% of patients experienced leukopenia; 42.4% experienced anemia; 3.0%, hepatic impairment; and 9.1%, gastrointestinal reactions.
Conclusion: Neoadjuvant combination therapy significantly enhances tumour response rates in HNSCC, with most patients with laryngeal cancer retaining laryngeal function. Adverse reactions remain clinically manageable, and the majority of patients tolerate the treatment well.
References
[1] Aboaid H, Khalid T, Hussain A, Myat YM, Nanda RK, Srinivasmurthy R, et al. (2025). Advances and challenges in immunotherapy in head and neck cancer. Front Immunol, 16, 1596583. https://doi.org/10.3389/fimmu.2025.1596583
[2] Liao C, An J, Tan Z, Xu F, Liu J, & Wang Q. (2021). Changes in Protein Glycosylation in Head and Neck Squamous Cell Carcinoma. J Cancer, 12(5), 1455-1466. https://doi.org/10.7150/jca.51604
[3] Ang MK, Montoya JE, Tharavichitkul E, Lim C, Tan T, Wang LY, et al. (2021). Phase II study of nimotuzumab (TheraCim-hR3) concurrent with cisplatin/radiotherapy in patients with locally advanced head and neck squamous cell carcinoma. Head Neck, 43(5), 1641-1651. https://doi.org/10.1002/hed.26635
[4] Abulizi A, Yan G, Xu Q, Muhetaer R, Wu S, Abudukelimu K, et al. (2024). Cardiovascular adverse events and immune-related adverse events associated with PD-1/PD-L1 inhibitors for head and neck squamous cell carcinoma (HNSCC). Sci Rep, 14(1), 25919. https://doi.org/10.1038/s41598-024-75099-5
[5] Fang Q, Li X, Xu P, Cao F, Wu D, Zhang X, et al. (2024). PD-1 inhibitor combined with paclitaxel and cisplatin in the treatment of recurrent and metastatic hypopharyngeal/laryngeal squamous cell carcinoma: efficacy and survival outcomes. Front Immunol, 15, 1353435. https://doi.org/10.3389/fimmu.2024.1353435
[6] Chen S, Yang Y, Wang R, & Fang J. (2023). Neoadjuvant PD-1/PD-L1 inhibitors combined with chemotherapy had a higher ORR than mono-immunotherapy in untreated HNSCC: Meta-analysis. Oral Oncol, 145, 106479. https://doi.org/10.1016/j.oraloncology.2023.106479
[7] Wang K, Gui L, Lu H, He X, Li D, Liu C, et al. (2023). Efficacy and safety of pembrolizumab with preoperative neoadjuvant chemotherapy in patients with resectable locally advanced head and neck squamous cell carcinomas. Front Immunol, 14, 1189752. https://doi.org/10.3389/fimmu.2023.1189752
[8] Liu C, Li M, Liu X, Shi T, Wang Y, Sui C, et al. (2024). Evaluating the efficacy and safety of different neoadjuvant immunotherapy combinations in locally advanced HNSCC: a systematic review and meta-analysis. Front Immunol, 15, 1467306. https://doi.org/10.3389/fimmu.2024.1467306
[9] Xue Y, Gao S, Gou J, Yin T, He H, Wang Y, et al. (2021). Platinum-based chemotherapy in combination with PD-1/PD-L1 inhibitors: preclinical and clinical studies and mechanism of action. Expert Opin Drug Deliv, 18(2), 187-203. https://doi.org/10.1080/17425247.2021.1825376
[10] Leidner R, Crittenden M, Young K, Xiao H, Wu Y, Couey MA, et al. (2021). Neoadjuvant immunoradiotherapy results in high rate of complete pathological response and clinical to pathological downstaging in locally advanced head and neck squamous cell carcinoma. J Immunother Cancer, 9(5). https://doi.org/10.1136/jitc-2021-002485
[11] Braakhuis BJ, Brakenhoff RH, & Leemans CR. (2012). Treatment choice for locally advanced head and neck cancers on the basis of risk factors: biological risk factors. Ann Oncol, 23 Suppl 10, x173-177. https://doi.org/10.1093/annonc/mds299
[12] Patel U, Kannan S, Rane SU, Mittal N, Gera P, Patil A, et al. (2022). Prognostic and predictive roles of cancer stem cell markers in head and neck squamous cell carcinoma patients receiving chemoradiotherapy with or without nimotuzumab. Br J Cancer, 126(10), 1439-1449. https://doi.org/10.1038/s41416-022-01730-9
[13] Gili R, Morbini P, & Bossi P. (2025). PD-L1 Expression in head and neck squamous cell carcinoma: qualitative or quantitative assessment? Is that enough or we need something more? Oral Oncol, 168, 107606. https://doi.org/10.1016/j.oraloncology.2025.107606
[14] Menon N, Patil V, Noronha V, Joshi A, Bhattacharjee A, Satam BJ, et al. (2021). Quality of life in patients with locally advanced head and neck cancer treated with concurrent chemoradiation with cisplatin and nimotuzumab versus cisplatin alone - Additional data from a phase 3 trial. Oral Oncol, 122, 105517. https://doi.org/10.1016/j.oraloncology.2021.105517
[15] Kejamurthy P, & Devi KTR. (2023). Immune checkpoint inhibitors and cancer immunotherapy by aptamers: an overview. Med Oncol, 41(1), 40. https://doi.org/10.1007/s12032-023-02267-4
[16] Daste A, Larroquette M, Gibson N, Lasserre M, & Domblides C. (2024). Immunotherapy for head and neck squamous cell carcinoma: current status and perspectives. Immunotherapy, 16(3), 187-197. https://doi.org/10.2217/imt-2023-0174
Type
Published
Issue
Section
License
Copyright (c) 2025 Head and Neck Diseases Conflux

This work is licensed under a Creative Commons Attribution 4.0 International License.







