Neuroimaging Advances in Neuropsychiatric Symptoms Associated with Parkinson’s Disease
DOI:
https://doi.org/10.71321/z4638252Keywords:
Neuroimaging, Parkinson’s DiseaseAbstract
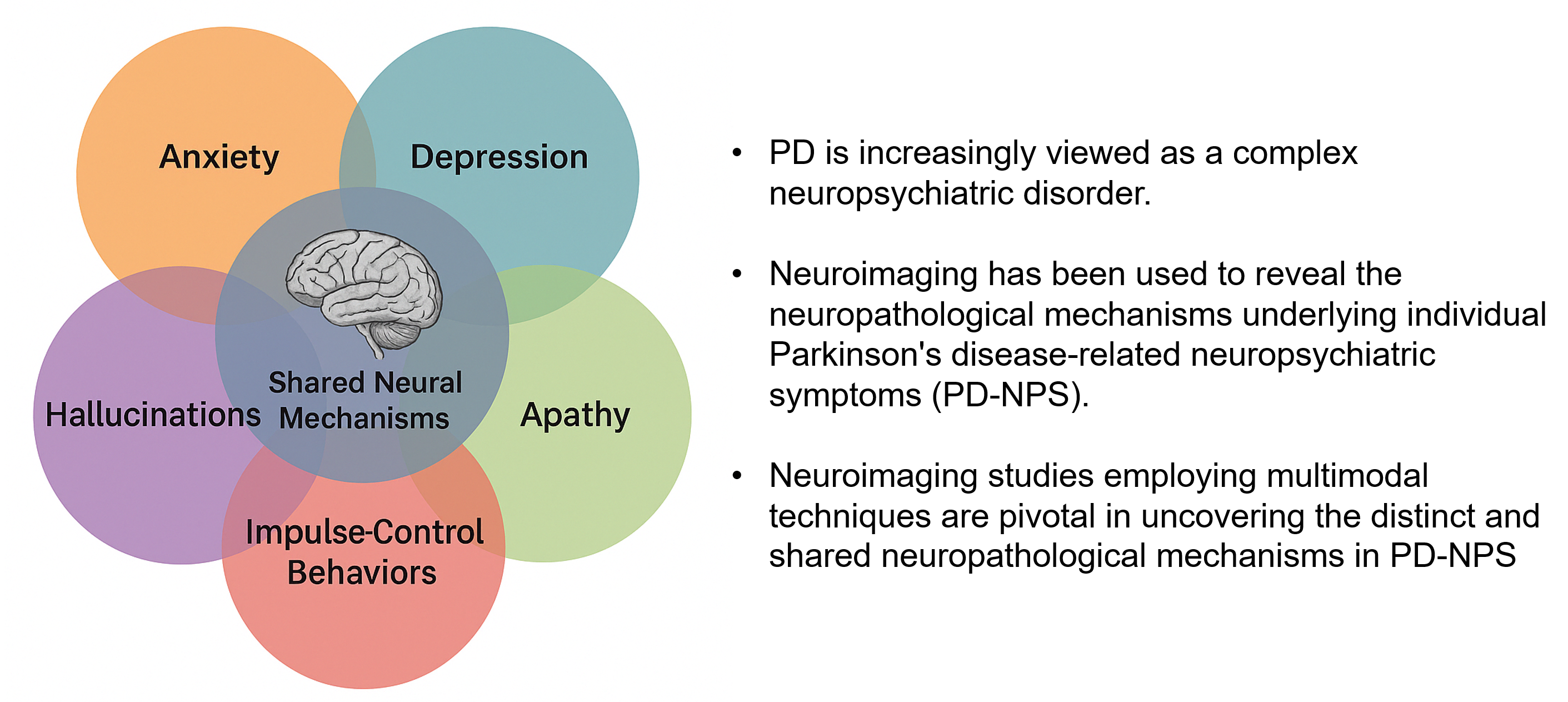
Parkinson’s disease (PD) is a prevalent neurodegenerative disorder traditionally defined by its motor symptoms yet increasingly recognized for its wide spectrum of neuropsychiatric symptoms (NPS) including anxiety, depression, apathy, impulse-control behaviors, and hallucinations. Recent neuroimaging advances have provided crucial insights into the neural substrates underlying these comorbidities. Structural imaging studies, using voxel-based morphometry and T1-weighted magnetic resonance imaging, have revealed regional atrophy in the frontal cortex, striatum, limbic areas, and occipital regions. In comparison, functional imaging using positron emission tomography, single-photon emission computed tomography, and resting-state functional MRI have identified abnormal network connectivity in circuits implicated in fear processing, reward regulation, and cognitive control. Overall, these imaging studies suggest shared and distinct pathophysiology of PD-related NPS, emphasizing the need for longitudinal, multimodal investigations to inform targeted therapeutic strategies and to improve clinical outcomes.
References
[1] Tolosa E, Garrido A, Scholz SW, Poewe W. Challenges in the diagnosis of Parkinson's disease. Lancet Neurol 2021;20:385-397.
[2] Ben-Shlomo Y, Darweesh S, Llibre-Guerra J, Marras C, San Luciano M, Tanner C. The epidemiology of Parkinson's disease. Lancet 2024;403:283-292.
[3] Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers 2017;3:17013.
[4] Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov Disord 2014;29:1583-1590.
[5] Alcalay RN, Caccappolo E, Mejia-Santana H, et al. Frequency of known mutations in early-onset Parkinson disease: implication for genetic counseling: the consortium on risk for early onset Parkinson disease study. Arch Neurol 2010;67:1116-1122.
[6] Marder KS, Tang MX, Mejia-Santana H, et al. Predictors of parkin mutations in early-onset Parkinson disease: the consortium on risk for early-onset Parkinson disease study. Arch Neurol 2010;67:731-738.
[7] Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol 2009;8:464-474.
[8] Weintraub D, Aarsland D, Chaudhuri KR, et al. The neuropsychiatry of Parkinson's disease: advances and challenges. Lancet Neurol 2022;21:89-102.
[9] Costello H, Schrag AE, Howard R, Roiser JP. Dissociable effects of dopaminergic medications on depression symptom dimensions in Parkinson disease. Nat Ment Health 2024;2:916-923.
[10] Aarsland D, Bronnick K, Alves G, et al. The spectrum of neuropsychiatric symptoms in patients with early untreated Parkinson's disease. J Neurol Neurosurg Psychiatry 2009;80:928-930.
[11] Kim R, Shin JH, Park S, Kim HJ, Jeon B. Longitudinal evolution of non-motor symptoms according to age at onset in early Parkinson's disease. J Neurol Sci 2020;418:117157.
[12] Ou R, Lin J, Liu K, et al. Evolution of Apathy in Early Parkinson's Disease: A 4-Years Prospective Cohort Study. Front Aging Neurosci 2020;12:620762.
[13] Yamamoto H. [Immunoglobulins in periapical lesion with special reference to qualitative and quantitative analysis of IgG]. Tsurumi Shigaku 1989;15:27-47.
[14] Broen MP, Narayen NE, Kuijf ML, Dissanayaka NN, Leentjens AF. Prevalence of anxiety in Parkinson's disease: A systematic review and meta-analysis. Mov Disord 2016;31:1125-1133.
[15] Pagonabarraga J, Martinez-Horta S, Fernandez de Bobadilla R, et al. Minor hallucinations occur in drug-naive Parkinson's disease patients, even from the premotor phase. Mov Disord 2016;31:45-52.
[16] Fenelon G, Soulas T, Zenasni F, Cleret de Langavant L. The changing face of Parkinson's disease-associated psychosis: a cross-sectional study based on the new NINDS-NIMH criteria. Mov Disord 2010;25:763-766.
[17] Dlay JK, Duncan GW, Khoo TK, et al. Progression of Neuropsychiatric Symptoms over Time in an Incident Parkinson's Disease Cohort (ICICLE-PD). Brain Sci 2020;10.
[18] Barrell K, Bureau B, Turcano P, et al. High-Order Visual Processing, Visual Symptoms, and Visual Hallucinations: A Possible Symptomatic Progression of Parkinson's Disease. Front Neurol 2018;9:999.
[19] Santangelo G, Trojano L, Barone P, Errico D, Grossi D, Vitale C. Apathy in Parkinson's disease: diagnosis, neuropsychological correlates, pathophysiology and treatment. Behav Neurol 2013;27:501-513.
[20] den Brok MG, van Dalen JW, van Gool WA, Moll van Charante EP, de Bie RM, Richard E. Apathy in Parkinson's disease: A systematic review and meta-analysis. Mov Disord 2015;30:759-769.
[21] Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol 2010;67:589-595.
[22] Markovic V, Stankovic I, Petrovic I, et al. Dynamics of impulsive-compulsive behaviors in early Parkinson's disease: a prospective study. J Neurol 2020;267:1127-1136.
[23] Corvol JC, Artaud F, Cormier-Dequaire F, et al. Longitudinal analysis of impulse control disorders in Parkinson disease. Neurology 2018;91:e189-e201.
[24] Gustafsson H, Nordstrom A, Nordstrom P. Depression and subsequent risk of Parkinson disease: A nationwide cohort study. Neurology 2015;84:2422-2429.
[25] Kazmi H, Walker Z, Booij J, et al. Late onset depression: dopaminergic deficit and clinical features of prodromal Parkinson's disease: a cross-sectional study. J Neurol Neurosurg Psychiatry 2021;92:158-164.
[26] Marinus J, Zhu K, Marras C, Aarsland D, van Hilten JJ. Risk factors for non-motor symptoms in Parkinson's disease. Lancet Neurol 2018;17:559-568.
[27] Hommel A, Meinders MJ, Lorenzl S, et al. The Prevalence and Determinants of Neuropsychiatric Symptoms in Late-Stage Parkinsonism. Mov Disord Clin Pract 2020;7:531-542.
[28] Weintraub D, Caspell-Garcia C, Simuni T, et al. Neuropsychiatric symptoms and cognitive abilities over the initial quinquennium of Parkinson disease. Ann Clin Transl Neurol 2020;7:449-461.
[29] Mueller C, Rajkumar AP, Wan YM, et al. Assessment and Management of Neuropsychiatric Symptoms in Parkinson's Disease. CNS Drugs 2018;32:621-635.
[30] Aarsland D, Marsh L, Schrag A. Neuropsychiatric symptoms in Parkinson's disease. Mov Disord 2009;24:2175-2186.
[31] Mamikonyan E, Siderowf AD, Duda JE, et al. Long-term follow-up of impulse control disorders in Parkinson's disease. Mov Disord 2008;23:75-80.
[32] Czernecki V, Pillon B, Houeto JL, Pochon JB, Levy R, Dubois B. Motivation, reward, and Parkinson's disease: influence of dopatherapy. Neuropsychologia 2002;40:2257-2267.
[33] Leentjens AF, Koester J, Fruh B, Shephard DT, Barone P, Houben JJ. The effect of pramipexole on mood and motivational symptoms in Parkinson's disease: a meta-analysis of placebo-controlled studies. Clin Ther 2009;31:89-98.
[34] Mills KA, Greene MC, Dezube R, Goodson C, Karmarkar T, Pontone GM. Efficacy and tolerability of antidepressants in Parkinson's disease: A systematic review and network meta-analysis. Int J Geriatr Psychiatry 2018;33:642-651.
[35] Kaji Y, Hirata K. Apathy and anhedonia in Parkinson's disease. ISRN Neurol 2011;2011:219427.
[36] Thomas A, Bonanni L, Gambi F, Di Iorio A, Onofrj M. Pathological gambling in Parkinson disease is reduced by amantadine. Ann Neurol 2010;68:400-404.
[37] Dobkin RD, Mann SL, Gara MA, Interian A, Rodriguez KM, Menza M. Telephone-based cognitive behavioral therapy for depression in Parkinson disease: A randomized controlled trial. Neurology 2020;94:e1764-e1773.
[38] Dobkin RD, Mann SL, Weintraub D, et al. Innovating Parkinson's Care: A Randomized Controlled Trial of Telemedicine Depression Treatment. Mov Disord 2021;36:2549-2558.
[39] Seppi K, Ray Chaudhuri K, Coelho M, et al. Update on treatments for nonmotor symptoms of Parkinson's disease-an evidence-based medicine review. Mov Disord 2019;34:180-198.
[40] Li S, Jiao R, Zhou X, Chen S. Motor recovery and antidepressant effects of repetitive transcranial magnetic stimulation on Parkinson disease: A PRISMA-compliant meta-analysis. Medicine (Baltimore) 2020;99:e19642.
[41] Lin F, Su Y, Weng Y, et al. The effects of bright light therapy on depression and sleep disturbances in patients with Parkinson's disease: a systematic review and meta-analysis of randomized controlled trials. Sleep Med 2021;83:280-289.
[42] Wu PL, Lee M, Huang TT. Effectiveness of physical activity on patients with depression and Parkinson's disease: A systematic review. PLoS One 2017;12:e0181515.
[43] Pontone GM, Williams JR, Anderson KE, et al. Anxiety and self-perceived health status in Parkinson's disease. Parkinsonism Relat Disord 2011;17:249-254.
[44] Leentjens AF, Dujardin K, Marsh L, Martinez-Martin P, Richard IH, Starkstein SE. Symptomatology and markers of anxiety disorders in Parkinson's disease: a cross-sectional study. Mov Disord 2011;26:484-492.
[45] Leentjens AF, Dujardin K, Marsh L, Martinez-Martin P, Richard IH, Starkstein SE. Anxiety and motor fluctuations in Parkinson's disease: a cross-sectional observational study. Parkinsonism Relat Disord 2012;18:1084-1088.
[46] Carey G, Gormezoglu M, de Jong JJA, et al. Neuroimaging of Anxiety in Parkinson's Disease: A Systematic Review. Mov Disord 2021;36:327-339.
[47] Volkmann J, Daniels C, Witt K. Neuropsychiatric effects of subthalamic neurostimulation in Parkinson disease. Nat Rev Neurol 2010;6:487-498.
[48] Thobois S, Prange S, Sgambato-Faure V, Tremblay L, Broussolle E. Imaging the Etiology of Apathy, Anxiety, and Depression in Parkinson's Disease: Implication for Treatment. Curr Neurol Neurosci Rep 2017;17:76.
[49] Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988;56:893-897.
[50] Vriend C, Boedhoe PS, Rutten S, Berendse HW, van der Werf YD, van den Heuvel OA. A smaller amygdala is associated with anxiety in Parkinson's disease: a combined FreeSurfer-VBM study. J Neurol Neurosurg Psychiatry 2016;87:493-500.
[51] Wee N, Wen MC, Kandiah N, et al. Neural correlates of anxiety symptoms in mild Parkinson's disease: A prospective longitudinal voxel-based morphometry study. J Neurol Sci 2016;371:131-136.
[52] Ma X, Su W, Li S, et al. Cerebellar atrophy in different subtypes of Parkinson's disease. J Neurol Sci 2018;392:105-112.
[53] Oosterwijk CS, Vriend C, Berendse HW, van der Werf YD, van den Heuvel OA. Anxiety in Parkinson's disease is associated with reduced structural covariance of the striatum. J Affect Disord 2018;240:113-120.
[54] Wang X, Li J, Wang M, et al. Alterations of the amplitude of low-frequency fluctuations in anxiety in Parkinson's disease. Neurosci Lett 2018;668:19-23.
[55] Zhang H, Qiu Y, Luo Y, et al. The relationship of anxious and depressive symptoms in Parkinson's disease with voxel-based neuroanatomical and functional connectivity measures. J Affect Disord 2019;245:580-588.
[56] Wang X, Li J, Yuan Y, et al. Altered putamen functional connectivity is associated with anxiety disorder in Parkinson's disease. Oncotarget 2017;8:81377-81386.
[57] Dan R, Ruzicka F, Bezdicek O, et al. Separate neural representations of depression, anxiety and apathy in Parkinson's disease. Sci Rep 2017;7:12164.
[58] Picillo M, Santangelo G, Erro R, et al. Association between dopaminergic dysfunction and anxiety in de novo Parkinson's disease. Parkinsonism Relat Disord 2017;37:106-110.
[59] Joling M, van den Heuvel OA, Berendse HW, Booij J, Vriend C. Serotonin transporter binding and anxiety symptoms in Parkinson's disease. J Neurol Neurosurg Psychiatry 2018;89:89-94.
[60] Bayram E, Kaplan N, Shan G, Caldwell JZK. The longitudinal associations between cognition, mood and striatal dopaminergic binding in Parkinson's Disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2020;27:581-594.
[61] Maillet A, Metereau E, Tremblay L, et al. Serotonergic and Dopaminergic Lesions Underlying Parkinsonian Neuropsychiatric Signs. Mov Disord 2021;36:2888-2900.
[62] Wen MC, Chan LL, Tan LC, Tan EK. Depression, anxiety, and apathy in Parkinson's disease: insights from neuroimaging studies. Eur J Neurol 2016;23:1001-1019.
[63] Huang C, Ravdin LD, Nirenberg MJ, et al. Neuroimaging markers of motor and nonmotor features of Parkinson's disease: an 18f fluorodeoxyglucose positron emission computed tomography study. Dement Geriatr Cogn Disord 2013;35:183-196.
[64] Ceravolo R, Frosini D, Poletti M, et al. Mild affective symptoms in de novo Parkinson's disease patients: relationship with dopaminergic dysfunction. Eur J Neurol 2013;20:480-485.
[65] Huang P, Xuan M, Gu Q, et al. Abnormal amygdala function in Parkinson's disease patients and its relationship to depression. J Affect Disord 2015;183:263-268.
[66] Mayberg HS, Starkstein SE, Sadzot B, et al. Selective hypometabolism in the inferior frontal lobe in depressed patients with Parkinson's disease. Ann Neurol 1990;28:57-64.
[67] Mentis MJ, McIntosh AR, Perrine K, et al. Relationships among the metabolic patterns that correlate with mnemonic, visuospatial, and mood symptoms in Parkinson's disease. Am J Psychiatry 2002;159:746-754.
[68] Badenoch JB, Paris A, Jacobs BM, Noyce AJ, Marshall CR, Waters S. Neuroanatomical and prognostic associations of depression in Parkinson's disease. J Neurol Neurosurg Psychiatry 2024;95:966-973.
[69] Yuan J, Liu Y, Liao H, et al. Alterations in cortical volume and complexity in Parkinson's disease with depression. CNS Neurosci Ther 2024;30:e14582.
[70] Feldmann A, Illes Z, Kosztolanyi P, et al. Morphometric changes of gray matter in Parkinson's disease with depression: a voxel-based morphometry study. Mov Disord 2008;23:42-46.
[71] Kostic VS, Agosta F, Petrovic I, et al. Regional patterns of brain tissue loss associated with depression in Parkinson disease. Neurology 2010;75:857-863.
[72] Deng X, Tang CY, Zhang J, et al. The cortical thickness correlates of clinical manifestations in the mid-stage sporadic Parkinson's disease. Neurosci Lett 2016;633:279-289.
[73] Surdhar I, Gee M, Bouchard T, Coupland N, Malykhin N, Camicioli R. Intact limbic-prefrontal connections and reduced amygdala volumes in Parkinson's disease with mild depressive symptoms. Parkinsonism Relat Disord 2012;18:809-813.
[74] Huang P, Xu X, Gu Q, et al. Disrupted white matter integrity in depressed versus non-depressed Parkinson's disease patients: a tract-based spatial statistics study. J Neurol Sci 2014;346:145-148.
[75] Li W, Liu J, Skidmore F, Liu Y, Tian J, Li K. White matter microstructure changes in the thalamus in Parkinson disease with depression: A diffusion tensor MR imaging study. AJNR Am J Neuroradiol 2010;31:1861-1866.
[76] Salehi MA, Mohammadi S, Gouravani M, Javidi A, Dager SR. Brain microstructural alterations of depression in Parkinson's disease: A systematic review of diffusion tensor imaging studies. Hum Brain Mapp 2022;43:5658-5680.
[77] Hu X, Song X, Yuan Y, et al. Abnormal functional connectivity of the amygdala is associated with depression in Parkinson's disease. Mov Disord 2015;30:238-244.
[78] Lou Y, Huang P, Li D, et al. Altered brain network centrality in depressed Parkinson's disease patients. Mov Disord 2015;30:1777-1784.
[79] Carriere N, Besson P, Dujardin K, et al. Apathy in Parkinson's disease is associated with nucleus accumbens atrophy: a magnetic resonance imaging shape analysis. Mov Disord 2014;29:897-903.
[80] Alzahrani H, Antonini A, Venneri A. Apathy in Mild Parkinson's Disease: Neuropsychological and Neuroimaging Evidence. J Parkinsons Dis 2016;6:821-832.
[81] Theis H, Prange S, Bischof GN, et al. Impulsive-compulsive behaviour in early Parkinson's disease is determined by apathy and dopamine receptor D3 polymorphism. NPJ Parkinsons Dis 2023;9:154.
[82] Ge S, Liu J, Jia Y, Li Z, Wang J, Wang M. Topological alteration of the brain structural network in Parkinson's disease with apathy. Brain Res Bull 2024;208:110899.
[83] Baggio HC, Segura B, Garrido-Millan JL, et al. Resting-state frontostriatal functional connectivity in Parkinson's disease-related apathy. Mov Disord 2015;30:671-679.
[84] Lucas-Jimenez O, Ojeda N, Pena J, et al. Apathy and brain alterations in Parkinson's disease: a multimodal imaging study. Ann Clin Transl Neurol 2018;5:803-814.
[85] Robert G, Le Jeune F, Lozachmeur C, et al. Apathy in patients with Parkinson disease without dementia or depression: a PET study. Neurology 2012;79:1155-1160.
[86] Robert GH, Le Jeune F, Lozachmeur C, et al. Preoperative factors of apathy in subthalamic stimulated Parkinson disease: a PET study. Neurology 2014;83:1620-1626.
[87] Zoon TJC, Mathiopoulou V, van Rooijen G, et al. Apathy following deep brain stimulation in Parkinson's disease visualized by 7-Tesla MRI subthalamic network analysis. Brain Stimul 2023;16:1289-1291.
[88] Boon LI, Potters WV, Zoon TJC, et al. Structural and functional correlates of subthalamic deep brain stimulation-induced apathy in Parkinson's disease. Brain Stimul 2021;14:192-201.
[89] Prange S, Metereau E, Maillet A, et al. Limbic Serotonergic Plasticity Contributes to the Compensation of Apathy in Early Parkinson's Disease. Mov Disord 2022;37:1211-1221.
[90] Voon V, Napier TC, Frank MJ, et al. Impulse control disorders and levodopa-induced dyskinesias in Parkinson's disease: an update. Lancet Neurol 2017;16:238-250.
[91] Biundo R, Weis L, Facchini S, et al. Patterns of cortical thickness associated with impulse control disorders in Parkinson's disease. Mov Disord 2015;30:688-695.
[92] Cerasa A, Salsone M, Nigro S, et al. Cortical volume and folding abnormalities in Parkinson's disease patients with pathological gambling. Parkinsonism Relat Disord 2014;20:1209-1214.
[93] Pellicano C, Niccolini F, Wu K, et al. Morphometric changes in the reward system of Parkinson's disease patients with impulse control disorders. J Neurol 2015;262:2653-2661.
[94] Tessitore A, Santangelo G, De Micco R, et al. Cortical thickness changes in patients with Parkinson's disease and impulse control disorders. Parkinsonism Relat Disord 2016;24:119-125.
[95] Petersen K, Van Wouwe N, Stark A, et al. Ventral striatal network connectivity reflects reward learning and behavior in patients with Parkinson's disease. Hum Brain Mapp 2018;39:509-521.
[96] Carriere N, Lopes R, Defebvre L, Delmaire C, Dujardin K. Impaired corticostriatal connectivity in impulse control disorders in Parkinson disease. Neurology 2015;84:2116-2123.
[97] Maggi G, Loayza F, Vitale C, Santangelo G, Obeso I. Anatomical correlates of apathy and impulsivity co-occurrence in early Parkinson's disease. J Neurol 2024;271:2798-2809.
[98] Gescheidt T, Marecek R, Mikl M, et al. Functional anatomy of outcome evaluation during Iowa Gambling Task performance in patients with Parkinson's disease: an fMRI study. Neurol Sci 2013;34:2159-2166.
[99] Frosini D, Pesaresi I, Cosottini M, et al. Parkinson's disease and pathological gambling: results from a functional MRI study. Mov Disord 2010;25:2449-2453.
[100] van der Vegt JP, Hulme OJ, Zittel S, et al. Attenuated neural response to gamble outcomes in drug-naive patients with Parkinson's disease. Brain 2013;136:1192-1203.
[101] Tessitore A, De Micco R, Giordano A, et al. Intrinsic brain connectivity predicts impulse control disorders in patients with Parkinson's disease. Mov Disord 2017;32:1710-1719.
[102] Fenelon G, Alves G. Epidemiology of psychosis in Parkinson's disease. J Neurol Sci 2010;289:12-17.
[103] Carter R, Ffytche DH. On visual hallucinations and cortical networks: a trans-diagnostic review. J Neurol 2015;262:1780-1790.
[104] Lenka A, Jhunjhunwala KR, Saini J, Pal PK. Structural and functional neuroimaging in patients with Parkinson's disease and visual hallucinations: A critical review. Parkinsonism Relat Disord 2015;21:683-691.
[105] Ozawa M, Shiraishi T, Murakami H, et al. Structural MRI study of Pareidolia and Visual Hallucinations in Drug-Naive Parkinson's disease. Sci Rep 2024;14:31293.
[106] Bhome R, Thomas GEC, Zarkali A, Weil RS. Structural and Functional Imaging Correlates of Visual Hallucinations in Parkinson's Disease. Curr Neurol Neurosci Rep 2023;23:287-299.
[107] Watanabe H, Senda J, Kato S, et al. Cortical and subcortical brain atrophy in Parkinson's disease with visual hallucination. Mov Disord 2013;28:1732-1736.
[108] Pezzoli S, Cagnin A, Bandmann O, Venneri A. Structural and Functional Neuroimaging of Visual Hallucinations in Lewy Body Disease: A Systematic Literature Review. Brain Sci 2017;7.
[109] Yao N, Cheung C, Pang S, et al. Multimodal MRI of the hippocampus in Parkinson's disease with visual hallucinations. Brain Struct Funct 2016;221:287-300.
[110] Goetz CG, Vaughan CL, Goldman JG, Stebbins GT. I finally see what you see: Parkinson's disease visual hallucinations captured with functional neuroimaging. Mov Disord 2014;29:115-117.
[111] Yao N, Pang S, Cheung C, et al. Resting activity in visual and corticostriatal pathways in Parkinson's disease with hallucinations. Parkinsonism Relat Disord 2015;21:131-137.
[112] Yao N, Shek-Kwan Chang R, Cheung C, et al. The default mode network is disrupted in Parkinson's disease with visual hallucinations. Hum Brain Mapp 2014;35:5658-5666.
[113] Maillet A, Krack P, Lhommee E, et al. The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson's disease. Brain 2016;139:2486-2502.
[114] Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson's disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 2005;128:1314-1322.
[115] Prange S, Metereau E, Maillet A, et al. Early limbic microstructural alterations in apathy and depression in de novo Parkinson's disease. Mov Disord 2019;34:1644-1654.
[116] Vriend C, Pattij T, van der Werf YD, et al. Depression and impulse control disorders in Parkinson's disease: two sides of the same coin? Neurosci Biobehav Rev 2014;38:60-71.
[117] Lu Q, Zhu Z, Zhang H, et al. Shared and distinct cortical morphometric alterations in five neuropsychiatric symptoms of Parkinson's disease. Transl Psychiatry 2024;14:347.
Type
Published
Data Availability Statement
Additional data related to this paper may be requested from the authors.
Issue
Section
License
Copyright (c) 2025 Brain Conflux

This work is licensed under a Creative Commons Attribution 4.0 International License.







