The cGAS-STING Pathway: Insights into Regulatory Mechanisms, Disease Dysregulation, and Therapeutic Development
DOI:
https://doi.org/10.71321/dr57c347Keywords:
cGAS, STING, Inflammation, Tumor Immunity, Diseases therapyAbstract
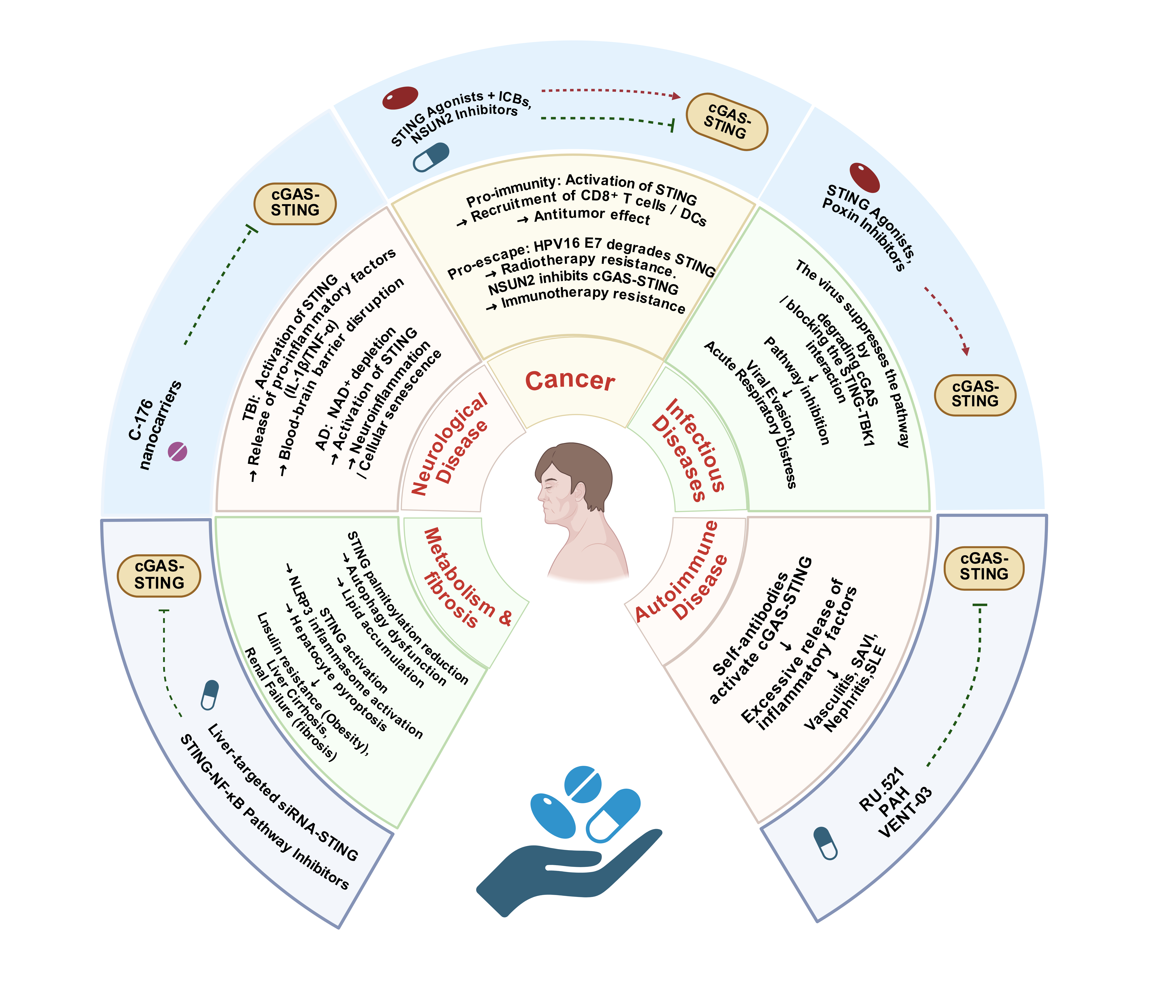
The cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway, a central hub of the innate immune system, is a key mediator of immune surveillance against abnormal cytoplasmic dsDNA: cGAS recognizes such dsDNA to synthesize 2'3'-cGAMP, which activates STING and downstream signaling to drive IFN-I and proinflammatory cytokine expression for the maintenance of homeostasis. This mechanism enables the pathway to exert multidimensional roles in physiology and pathology. Its activity is fine-tuned by post-translational modifications and non-coding RNAs. Given its critical role in linking innate immunity to disease progression, it has become a promising therapeutic target. This review summarizes the pathway’s regulatory mechanisms and pathological implications, detailing its roles in immune activation, disease dysregulation, and therapeutic development. It also addresses existing challenges and proposes future directions, aiming to provide new insights for precision therapy against cGAS-STING-associated diseases.
References
[1] Rousset F. (2023). Innate immunity: the bacterial connection. Trends Immunol, 44(12), 945-953. https://doi.org/10.1016/j.it.2023.10.001
[2] Xu Z, Kombe Kombe AJ, Deng S, Zhang H, Wu S, Ruan J, et al. (2024). NLRP inflammasomes in health and disease. Mol Biomed, 5(1), 14. https://doi.org/10.1186/s43556-024-00179-x
[3] Chen R, Zou J, Chen J, Zhong X, Kang R, & Tang D. (2025). Pattern recognition receptors: function, regulation and therapeutic potential. Signal Transduct Target Ther, 10(1), 216. https://doi.org/10.1038/s41392-025-02264-1
[4] Zhang X, Bai X, & Chen Z. (2020). Structures and mechanisms in the cGAS-STING innate immunity pathway. Immunity, 53(1), 43-53. https://doi.org/10.1016/j.immuni.2020.05.013
[5] Lin H, Pang W, Yuan H, Kong Y, Long F, Zhang R, et al. (2023). Molecular subtypes based on DNA sensors predict prognosis and tumor immunophenotype in hepatocellular carcinoma. Aging (Albany NY), 15(14), 6798. https://doi.org/10.18632/aging.204870
[6] Dvorkin S, Cambier S, Volkman HE, & Stetson DB. (2024). New frontiers in the cGAS-STING intracellular DNA-sensing pathway. Immunity, 57(4), 718-730. https://doi.org/10.1016/j.immuni.2024.02.019
[7] Pang W, Wang Y, Lu X, Li M, Long F, Chen S, et al. (2025). Integrated spatial and single cell transcriptomics identifies PRKDC as a dual prognostic biomarker and therapeutic target in hepatocellular carcinoma. Sci Rep, 15(1), 14834. https://doi.org/10.1038/s41598-025-98866-4
[8] Zhang B, Xu P, & Ablasser A. (2025). Regulation of the cGAS-STING Pathway. Annu Rev Immunol, 43(1), 667-692. https://doi.org/10.1146/annurev-immunol-101721-032910
[9] Chauvin SD, Stinson WA, Platt DJ, Poddar S, & Miner J. (2023). Regulation of cGAS and STING signaling during inflammation and infection. J Biol Chem, 299(7), 104866. https://doi.org/10.1016/j.jbc.2023.104866
[10] Shen M, Jiang X, Peng Q, Oyang L, Ren Z, Wang J, et al. (2025). The cGAS‒STING pathway in cancer immunity: mechanisms, challenges, and therapeutic implications. J Hematol Oncol, 18(1), 40. https://doi.org/10.1186/s13045-025-01691-5
[11] Soh L, Lee S, Roebuck MM, & Wong P. (2024). Unravelling the interplay between ER stress, UPR and the cGAS-STING pathway: Implications for osteoarthritis pathogenesis and treatment strategy. Life Sci, 357, 123112. https://doi.org/10.1016/j.lfs.2024.123112
[12] Zhao B, Du F, Xu P, Shu C, Sankaran B, Bell SL, et al. (2019). A conserved PLPLRT/SD motif of STING mediates the recruitment and activation of TBK1. Nature, 569(7758), 718-722. https://doi.org/10.1038/s41586-019-1228-x
[13] Zhang Z, & Zhang C. (2025). Regulation of cGAS–STING signalling and its diversity of cellular outcomes. Nat Rev Immunol, 25(6), 425-444. https://doi.org/10.1038/s41577-024-01112-7
[14] Kong E, Hua T, Li J, Li Y, Yang M, Ding R, et al. (2024). HSV-1 reactivation results in post-herpetic neuralgia by upregulating Prmt6 and inhibiting cGAS-STING. Brain, 147(7), 2552-2565. https://doi.org/10.1093/brain/awae053
[15] Zhang H, Han C, Li T, Li N, & Cao X. (2019). The methyltransferase PRMT6 attenuates antiviral innate immunity by blocking TBK1–IRF3 signaling. Cell Mol Immunol, 16(10), 800-809. https://doi.org/10.1038/s41423-018-0057-4
[16] Decout A, Katz JD, Venkatraman S, & Ablasser A. (2021). The cGAS–STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol, 21(9), 548-569. https://doi.org/10.1038/s41577-021-00524-z
[17] Lama L, Adura C, Xie W, Tomita D, Kamei T, Kuryavyi V, et al. (2019). Development of human cGAS-specific small-molecule inhibitors for repression of dsDNA-triggered interferon expression. Nat Commun, 10(1), 2261. https://doi.org/10.1038/s41467-019-08620-4
[18] Wiser C, Kim B, Vincent J, & Ascano M. (2020). Small molecule inhibition of human cGAS reduces total cGAMP output and cytokine expression in cells. Sci Rep, 10(1), 7604. https://doi.org/10.1038/s41598-020-64348-y
[19] Yan X, Huang J, Zeng Y, Zhong X, Fu Y, Xiao H, et al. (2024). CGRP attenuates pulmonary vascular remodeling by inhibiting the cGAS-STING-NFκB pathway in pulmonary arterial hypertension. Biochem Pharmacol, 222, 116093. https://doi.org/10.1016/j.bcp.2024.116093
[20] Mullard A. (2023). Biotechs step on cGAS for autoimmune diseases. Nat Rev Drug Discov, 22(12), 939-941. https://doi.org/10.1038/d41573-023-00185-8
[21] Ishikawa H, & Barber GN. (2008). STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature, 455(7213), 674-678. https://doi.org/10.1038/nature07317
[22] Smith JA. (2021). STING, the endoplasmic reticulum, and mitochondria: is three a crowd or a conversation? Front Immunol, 11, 611347. https://doi.org/10.3389/fimmu.2020.611347
[23] Luo Y, Chang L, Ji Y, & Liang T. (2024). ER: a critical hub for STING signaling regulation. Trends Cell Biol, 34(10), 865-881. https://doi.org/10.1016/j.tcb.2024.02.006
[24] Xu Y, Wang Q, Wang J, Qian C, Wang Y, Lu S, et al. (2025). The cGAS-STING pathway activates transcription factor TFEB to stimulate lysosome biogenesis and pathogen clearance. Immunity, 58(2), 309-325. e306. https://doi.org/10.1016/j.immuni.2024.11.017
[25] Zhu H, Zhang R, Yi L, Tang Y, & Zheng C. (2022). UNC93B1 attenuates the cGAS–STING signaling pathway by targeting STING for autophagy–lysosome degradation. J Med Virol, 94(9), 4490-4501. https://doi.org/10.1002/jmv.27860
[26] Wu Y, Lin Y, Shen F, Huang R, Zhang Z, Zhou M, et al. (2024). FBXO38 deficiency promotes lysosome-dependent STING degradation and inhibits cGAS–STING pathway activation. Neoplasia, 49, 100973. https://doi.org/10.1016/j.neo.2024.100973
[27] Hancock Cerutti W, Wu Z, Xu P, Yadavalli N, Leonzino M, Tharkeshwar AK, et al. (2022). ER-lysosome lipid transfer protein VPS13C/PARK23 prevents aberrant mtDNA-dependent STING signaling. J Cell Biol, 221(7), e202106046. https://doi.org/10.1083/jcb.202106046
[28] Bustos MA, Yokoe T, Shoji Y, Kobayashi Y, Mizuno S, Murakami T, et al. (2023). MiR-181a targets STING to drive PARP inhibitor resistance in BRCA-mutated triple-negative breast cancer and ovarian cancer. Cell Biosci, 13(1), 200. https://doi.org/10.1186/s13578-023-01151-y
[29] Tian X, Zhang P, Liu F, Yang L, Fu K, Gan K, et al. (2023). MicroRNA‐4691‐3p inhibits the inflammatory response by targeting STING in human dental pulp cells: A laboratory investigation. Int Endod J, 56(11), 1328-1336. https://doi.org/10.1111/iej.13953
[30] Chen X, Wang L, Cheng Q, Deng Z, Tang Y, Yan Y, et al. (2024). Multiple myeloma exosomal miRNAs suppress cGAS-STING antiviral immunity. Biochim Biophys Acta Mol Basis Dis, 1870(8), 167457. https://doi.org/10.1016/j.bbadis.2024.167457
[31] Song J, Zhang L, Li C, Maimaiti M, Sun J, Hu J, et al. (2022). m6A-mediated modulation coupled with transcriptional regulation shapes long noncoding RNA repertoire of the cGAS-STING signaling. Comput Struct Biotechnol J, 20, 1785-1797. https://doi.org/10.1016/j.csbj.2022.04.002
[32] Gao Y, Zhang N, Zeng Z, Wu Q, Jiang X, Li S, et al. (2022). LncRNA PCAT1 activates SOX2 and suppresses radioimmune responses via regulating cGAS/STING signalling in non‐small cell lung cancer. Clin Transl Med, 12(4), e792. https://doi.org/10.1002/ctm2.792
[33] Zhuang Q, Liu C, Hu Y, Liu Y, Lyu Y, Liao Y, et al. (2023). Identification of RP11‐770J1. 4 as immune‐related lncRNA regulating the CTXN1–cGAS–STING axis in histologically lower‐grade glioma. MedComm, 4(6), e458. https://doi.org/10.1002/mco2.458
[34] Han J, Wang Y, Yang R, Xu Y, Li F, & Jia Y. (2022). LncRNA FAM225A activates the cGAS-STING signaling pathway by combining FUS to promote CENP-N expression and regulates the progression of nasopharyngeal carcinoma. Pathol Res Pract, 236, 154005. https://doi.org/10.1016/j.prp.2022.154005
[35] Gu Y, Lv L, Jin J, Hua X, Xu Q, Wu R, et al. (2024). STING mediates LPS-induced acute lung injury by regulating ferroptosis. Exp Cell Res, 438(2), 114039. https://doi.org/10.1016/j.yexcr.2024.114039
[36] Erttmann SF, Swacha P, Aung KM, Brindefalk B, Jiang H, Härtlova A, et al. (2022). The gut microbiota prime systemic antiviral immunity via the cGAS-STING-IFN-I axis. Immunity, 55(5), 847-861. e810. https://doi.org/10.1016/j.immuni.2022.04.006
[37] Gui X, Yang H, Li T, Tan X, Shi P, Li M, et al. (2019). Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature, 567(7747), 262-266. https://doi.org/10.1038/s41586-019-1006-9
[38] Prabakaran T, Bodda C, Krapp C, Zhang Bc, Christensen MH, Sun C, et al. (2018). Attenuation of c GAS‐STING signaling is mediated by a p62/SQSTM 1‐dependent autophagy pathway activated by TBK1. EMBO J, 37(8), e97858. https://doi.org/10.15252/embj.201797858
[39] Luo S, Luo R, Lu H, Zhang R, Deng G, Luo H, et al. (2023). Activation of cGAS-STING signaling pathway promotes liver fibrosis and hepatic sinusoidal microthrombosis. Int Immunopharmacol, 125, 111132. https://doi.org/10.1016/j.intimp.2023.111132
[40] Hu B, Ma J, & Duerfeldt AS. (2023). The cGAS–STING pathway in diabetic retinopathy and age-related macular degeneration. Future Med Chem, 15(8), 717-729. https://doi.org/10.4155/fmc-2022-0301
[41] Wottawa F, Bordoni D, Baran N, Rosenstiel P, & Aden K. (2021). The role of cGAS/STING in intestinal immunity. Eur J Immunol, 51(4), 785-797. https://doi.org/10.1002/eji.202048777
[42] Frémond ML, Hadchouel A, Berteloot L, Melki I, Bresson V, Barnabei L, et al. (2021). Overview of STING-associated vasculopathy with onset in infancy (SAVI) among 21 patients. J Allergy Clin Immunol Pract, 9(2), 803-818. e811. https://doi.org/10.1016/j.jaip.2020.11.007
[43] Lin B, Berard R, Al Rasheed A, Aladba B, Kranzusch PJ, Henderlight M, et al. (2020). A novel STING1 variant causes a recessive form of STING-associated vasculopathy with onset in infancy (SAVI). J Allergy Clin Immunol, 146(5), 1204-1208. e1206. https://doi.org/10.1016/j.jaci.2020.06.032
[44] Frémond ML, Rodero MP, Jeremiah N, Belot A, Jeziorski E, Duffy D, et al. (2016). Efficacy of the Janus kinase 1/2 inhibitor ruxolitinib in the treatment of vasculopathy associated with TMEM173-activating mutations in 3 children. J Allergy Clin Immunol, 138(6), 1752-1755. https://doi.org/10.1016/j.jaci.2016.07.015
[45] Poddar S, Chauvin SD, Archer CH, Qian W, Castillo-Badillo JA, Yin X, et al. (2025). ArfGAP2 promotes STING proton channel activity, cytokine transit, and autoinflammation. Cell, 188(6), 1605-1622. e1626. https://doi.org/10.1016/j.cell.2025.01.027
[46] Hu Y, Chen B, Yang F, Su Y, Yang D, Yao Y, et al. (2022). Emerging role of the cGAS-STING signaling pathway in autoimmune diseases: Biologic function, mechanisms and clinical prospection. Autoimmun Rev, 21(9), 103155. https://doi.org/10.1016/j.autrev.2022.103155
[47] Jeremiah N, Neven B, Gentili M, Callebaut I, Maschalidi S, Stolzenberg MC, et al. (2014). Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest, 124(12), 5516-5520. https://doi.org/10.1172/JCI79100
[48] Feng Q, Xu X, & Zhang S. (2024). cGAS-STING pathway in systemic lupus erythematosus: biological implications and therapeutic opportunities. Immunol Res, 72(6), 1207-1216. https://doi.org/10.1007/s12026-024-09525-1
[49] Chen J, Chen P, Song Y, Wei J, Wu F, Sun J, et al. (2024). STING upregulation mediates ferroptosis and inflammatory response in lupus nephritis by upregulating TBK1 and activating NF-κB signal pathway. J Biosci, 49(1), 9. https://doi.org/10.1007/s12038-023-00381-z
[50] Motwani M, McGowan J, Antonovitch J, Gao KM, Jiang Z, Sharma S, et al. (2021). cGAS-STING pathway does not promote autoimmunity in murine models of SLE. Front Immunol, 12, 605930. https://doi.org/10.3389/fimmu.2021.605930
[51] Zhang X, He B, Lu J, Bao Q, Wang J, & Yang Y. (2024). The crucial roles and research advances of cGAS‑STING pathway in liver diseases. Ann Med, 56(1), 2394588. https://doi.org/10.1080/07853890.2024.2394588
[52] Maekawa H, Inoue T, Ouchi H, Jao TM, Inoue R, Nishi H, et al. (2019). Mitochondrial damage causes inflammation via cGAS-STING signaling in acute kidney injury. Cell Rep, 29(5), 1261-1273. e1266. https://doi.org/10.1016/j.celrep.2019.09.050
[53] Wu J, Raman A, Coffey NJ, Sheng X, Wahba J, Seasock MJ, et al. (2021). The key role of NLRP3 and STING in APOL1-associated podocytopathy. J Clin Invest, 131(20), e136329. https://doi.org/10.1172/JCI136329
[54] García Giménez J, Córdoba David G, Rayego Mateos S, Cannata Ortiz P, Carrasco S, Ruiz Ortega M, et al. (2023). STING1 deficiency ameliorates immune‐mediated crescentic glomerulonephritis in mice. J Pathol, 261(3), 309-322. https://doi.org/10.1002/path.6177
[55] Dong M, Chen M, Zhang Y, He X, Min J, Tan Y, et al. (2024). Oscillatory shear stress promotes endothelial senescence and atherosclerosis via STING activation. Biochem Biophys Res Commun, 715, 149979. https://doi.org/10.1016/j.bbrc.2024.149979
[56] Chen Q, Tang L, Zhang Y, Wan C, Yu X, Dong Y, et al. (2022). STING up-regulates VEGF expression in oxidative stress-induced senescence of retinal pigment epithelium via NF-κB/HIF-1α pathway. Life Sci, 293, 120089. https://doi.org/10.1016/j.lfs.2021.120089
[57] Yu H, Liao K, Hu Y, Lv D, Luo M, Liu Q, et al. (2022). Role of the cGAS-STING pathway in aging-related endothelial dysfunction. Aging Dis, 13(6), 1901. https://doi.org/10.14336/AD.2022.0316
[58] Hou Y, Wei Y, Lautrup S, Yang B, Wang Y, Cordonnier S, et al. (2021). NAD+ supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer’s disease via cGAS–STING. Proc Natl Acad Sci U S A, 118(37), e2011226118. https://doi.org/10.1073/pnas.2011226118
[59] Chan R, Cao X, Ergun SL, Njomen E, Lynch SR, Ritchie C, et al. (2025). Cysteine allostery and autoinhibition govern human STING oligomer functionality. Nat Chem Biol, 10.1038/s41589-025-01951-y, 1-10. https://doi.org/10.1038/s41589-025-01951-y
[60] Krawczyk E, Kangas C, & He B. (2023). HSV Replication: Triggering and repressing STING functionality. Viruses, 15(1), 226. https://doi.org/10.3390/v15010226
[61] Dogrammatzis C, Saud R, Waisner H, Lasnier S, Suma SM, Grieshaber B, et al. (2024). Tracing the STING exocytosis pathway during herpes viruses infection. mBio, 15(4), e00373-00324. https://doi.org/10.1128/mbio.00373-24
[62] Huang J, You H, Su C, Li Y, Chen S, & Zheng C. (2018). Herpes simplex virus 1 tegument protein VP22 abrogates cGAS/STING-mediated antiviral innate immunity. J Virol, 92(15), e00841-00818. https://doi.org/10.1128/JVI.00841-18
[63] Neufeldt CJ, Cerikan B, Cortese M, Frankish J, Lee J-Y, Plociennikowska A, et al. (2022). SARS-CoV-2 infection induces a pro-inflammatory cytokine response through cGAS-STING and NF-κB. Commun Biol, 5(1), 45. https://doi.org/10.1038/s42003-021-02983-5
[64] McKnight KL, Swanson KV, Austgen K, Richards C, Mitchell JK, McGivern DR, et al. (2020). Stimulator of interferon genes (STING) is an essential proviral host factor for human rhinovirus species A and C. Proc Natl Acad Sci U S A, 117(44), 27598-27607. https://doi.org/10.1073/pnas.2014940117
[65] Zhang B-c, Nandakumar R, Reinert LS, Huang J, Laustsen A, Gao Z-l, et al. (2020). STEEP mediates STING ER exit and activation of signaling. Nat Immunol, 21(8), 868-879. https://doi.org/10.1038/s41590-020-0730-5
[66] Gu Y, Lin S, Wu Y, Xu P, Zhu W, Wang Y, et al. (2023). Targeting STING activation by antigen‐Inspired MnO2 nanovaccines optimizes tumor radiotherapy. Adv Healthc Mater, 12(12), 2300028. https://doi.org/10.1002/adhm.202300028
[67] Yan Z, Yue J, Zhang Y, Hou Z, Li D, Yang Y, et al. (2024). Pseudorabies virus VHS protein abrogates interferon responses by blocking NF-κB and IRF3 nuclear translocation. Virol Sin, 39(4), 587-599. https://doi.org/10.1016/j.virs.2024.05.009
[68] Chen L, Hu H, Pan Y, Lu Y, Zhao M, Zhao Y, et al. (2024). The role of HPV11 E7 in modulating STING-dependent interferon β response in recurrent respiratory papillomatosis. J Virol, 98(5), e01925-01923. https://doi.org/10.1128/jvi.01925-23
[69] Huérfano S, Šroller V, Bruštíková K, Horníková L, & Forstová J. (2022). The interplay between viruses and host DNA sensors. Viruses, 14(4), 666. https://doi.org/10.3390/v14040666
[70] Yum S, Li M, Fang Y, & Chen Z. (2021). TBK1 recruitment to STING activates both IRF3 and NF-κB that mediate immune defense against tumors and viral infections. Proc Natl Acad Sci U S A, 118(14), e2100225118. https://doi.org/10.1073/pnas.2100225118
[71] Tu Y, Zhu Q, Huang W, Feng S, Tan Y, Li L, et al. (2025). DNMT inhibition epigenetically restores the cGAS-STING pathway and activates RIG-I/MDA5-MAVS to enhance antitumor immunity. Acta Pharmacol Sin, 10.1038/s41401-025-01639-y, 1-12. https://doi.org/10.1038/s41401-025-01639-y
[72] Ruiz-Moreno JS, Hamann L, Shah JA, Verbon A, Mockenhaupt FP, Puzianowska Kuznicka M, et al. (2018). The common HAQ STING variant impairs cGAS-dependent antibacterial responses and is associated with susceptibility to Legionnaires’ disease in humans. PLoS Pathog, 14(1), e1006829. https://doi.org/10.1371/journal.ppat.1006829
[73] Bishop RC, & Derré I. (2022). The Chlamydia trachomatis inclusion membrane protein CTL0390 mediates host cell exit via lysis through STING activation. Infect Immun, 90(6), e00190-00122. https://doi.org/10.1128/iai.00190-22
[74] Ying T, Yu Y, Yu Q, Zhou G, Chen L, Gu Y, et al. (2024). The involvement of Sting in exacerbating acute lung injury in sepsis via the PARP-1/NLRP3 signaling pathway. Pulm Pharmacol Ther, 86, 102303. https://doi.org/10.1016/j.pupt.2024.102303
[75] Tao Y, Yin S, Liu Y, Li C, Chen Y, Han D, et al. (2023). UFL1 promotes antiviral immune response by maintaining STING stability independent of UFMylation. Cell Death Differ, 30(1), 16-26. https://doi.org/10.1038/s41418-022-01041-9
[76] Wang Y, Rivera FDL, Edwards CL, Frame TC, Engel JA, Bukali L, et al. (2023). STING activation promotes autologous type I interferon–dependent development of type 1 regulatory T cells during malaria. J Clin Invest, 133(19), e169417. https://doi.org/10.1172/JCI169417
[77] Nitta S, Sakamoto N, Nakagawa M, Kakinuma S, Mishima K, Kusano‐Kitazume A, et al. (2013). Hepatitis C virus NS4B protein targets STING and abrogates RIG‐I–mediated type I interferon‐dependent innate immunity. Hepatology, 57(1), 46-58. https://doi.org/10.1002/hep.26017
[78] Losarwar S, Pancholi B, Babu R, & Garabadu D. (2025). Mitochondria-dependent innate immunity: A potential therapeutic target in Flavivirus infection. Int Immunopharmacol, 154, 114551. https://doi.org/10.1016/j.intimp.2025.114551
[79] AlDaif BA, Mercer AA, & Fleming SB. (2023). The parapoxvirus Orf virus inhibits dsDNA-mediated type I IFN expression via STING-dependent and STING-independent signalling pathways. J Gen Virol, 104(10), 001912. https://doi.org/10.1099/jgv.0.001912
[80] Georgana I, Sumner RP, Towers GJ, & Maluquer de Motes C. (2018). Virulent poxviruses inhibit DNA sensing by preventing STING activation. J Virol, 92(10), e02145-02117. https://doi.org/10.1128/JVI.02145-17
[81] Ruiz Moreno JS, Hamann L, Jin L, Sander LE, Puzianowska-Kuznicka M, Cambier J, et al. (2018). The cGAS/STING pathway detects Streptococcus pneumoniae but appears dispensable for antipneumococcal defense in mice and humans. Infect Immun, 86(3), e00849-00817. https://doi.org/10.1128/IAI.00849-17
[82] Nissen SK, Pedersen JG, Helleberg M, Kjær K, Thavachelvam K, Obel N, et al. (2018). Multiple homozygous variants in the STING-encoding TMEM173 gene in HIV long-term nonprogressors. J Immunol, 200(10), 3372-3382. https://doi.org/10.4049/jimmunol.1701284
[83] de Lima LLP, de Oliveira AQT, Moura TCF, da Silva Graça Amoras E, Lima SS, da Silva ANMR, et al. (2021). STING and cGAS gene expressions were downregulated among HIV-1-infected persons after antiretroviral therapy. Virol J, 18(1), 78. https://doi.org/10.1186/s12985-021-01548-6
[84] Chan P, Ye ZW, Zhao W, Ong CP, Sun XY, Cheung PHH, et al. (2025). Mpox virus poxin-schlafen fusion protein suppresses innate antiviral response by sequestering STAT2. Emerg Microbes Infect, 14(1), 2477639. https://doi.org/10.1080/22221751.2025.2477639
[85] Huang R, Ning Q, Zhao J, Zhao X, Zeng L, Yi Y, et al. (2022). Targeting STING for cancer immunotherapy: From mechanisms to translation. Int Immunopharmacol, 113, 109304. https://doi.org/10.1016/j.intimp.2022.109304
[86] Varga KZ, Gyurina K, Radványi Á, Pál T, Sasi-Szabó L, Yu H, et al. (2023). Stimulator of interferon genes (STING) triggers adipocyte autophagy. Cells, 12(19), 2345. https://doi.org/10.3390/cells12192345
[87] Sebastian M, Hsiao CJ, Futch HS, Eisinger RS, Dumeny L, Patel S, et al. (2020). Obesity and STING1 genotype associate with 23-valent pneumococcal vaccination efficacy. JCI Insight, 5(9), e136141. https://doi.org/10.1172/jci.insight.136141
[88] Kang J, Wu J, Liu Q, Jiang H, Li W, Li Y, et al. (2024). FASN regulates STING palmitoylation via malonyl-CoA in macrophages to alleviate sepsis-induced liver injury. Biochim Biophys Acta Mol Basis Dis, 1870(7), 167299. https://doi.org/10.1016/j.bbadis.2024.167299
[89] Mukai K, Konno H, Akiba T, Uemura T, Waguri S, Kobayashi T, et al. (2016). Activation of STING requires palmitoylation at the Golgi. Nat Commun, 7(1), 11932. https://doi.org/10.1038/ncomms11932
[90] Geng K, Ma X, Jiang Z, Huang W, Gu J, Wang P, et al. (2023). High glucose-induced STING activation inhibits diabetic wound healing through promoting M1 polarization of macrophages. Cell Death Discov, 9(1), 136. https://doi.org/10.1038/s41420-023-01425-x
[91] Qiao J, Zhang Z, Ji S, Liu T, Zhang X, Huang Y, et al. (2022). A distinct role of STING in regulating glucose homeostasis through insulin sensitivity and insulin secretion. Proc Natl Acad Sci U S A, 119(7), e2101848119. https://doi.org/10.1073/pnas.2101848119
[92] Chen T, Xu ZG, Luo J, Manne RK, Wang Z, Hsu CC, et al. (2023). NSUN2 is a glucose sensor suppressing cGAS/STING to maintain tumorigenesis and immunotherapy resistance. Cell Metab, 35(10), 1782-1798. e1788. https://doi.org/10.1016/j.cmet.2023.07.009
[93] Wang X, Rao H, Zhao J, Wee A, Li X, Fei R, et al. (2020). STING expression in monocyte-derived macrophages is associated with the progression of liver inflammation and fibrosis in patients with nonalcoholic fatty liver disease. Lab Invest, 100(4), 542-552. https://doi.org/10.1038/s41374-019-0342-6
[94] Luo X, Li H, Ma L, Zhou J, Guo X, Woo S-L, et al. (2018). Expression of STING is increased in liver tissues from patients with NAFLD and promotes macrophage-mediated hepatic inflammation and fibrosis in mice. Gastroenterology, 155(6), 1971-1984. e1974. https://doi.org/10.1053/j.gastro.2018.09.010
[95] Xiao Y, Zhao C, Tai Y, Li B, Lan T, Lai E, et al. (2023). STING mediates hepatocyte pyroptosis in liver fibrosis by epigenetically activating the NLRP3 inflammasome. Redox Biol, 62, 102691. https://doi.org/10.1016/j.redox.2023.102691
[96] Zhang J, Zhang L, Chen Y, Fang X, Li B, & Mo C. (2023). The role of cGAS-STING signaling in pulmonary fibrosis and its therapeutic potential. Front Immunol, 14, 1273248. https://doi.org/10.3389/fimmu.2023.1273248
[97] Zhang J, Du J, Liu D, Zhuo J, Chu L, Li Y, et al. (2024). Polystyrene microplastics induce pulmonary fibrosis by promoting alveolar epithelial cell ferroptosis through cGAS/STING signaling. Ecotoxicol Environ Saf, 277, 116357. https://doi.org/10.1016/j.ecoenv.2024.116357
[98] Savigny F, Schricke C, Lacerda-Queiroz N, Meda M, Nascimento M, Huot-Marchand S, et al. (2021). Protective role of the nucleic acid sensor STING in pulmonary fibrosis. Front Immunol, 11, 588799. https://doi.org/10.3389/fimmu.2020.588799
[99] Gao L, Zhang J, Yang T, Jiang L, Liu X, Wang S, et al. (2023). STING/ACSL4 axis-dependent ferroptosis and inflammation promote hypertension-associated chronic kidney disease. Mol Ther, 31(10), 3084-3103. https://doi.org/10.1016/j.ymthe.2023.07.026
[100] Chung KW, Dhillon P, Huang S, Sheng X, Shrestha R, Qiu C, et al. (2019). Mitochondrial damage and activation of the STING pathway lead to renal inflammation and fibrosis. Cell Metab, 30(4), 784-799. e785. https://doi.org/10.1016/j.cmet.2019.08.003
[101] Tian X, Zeng Y, Tu Q, Jiao Y, Yao S, Chen Y, et al. (2023). Butyrate alleviates renal fibrosis in CKD by regulating NLRP3-mediated pyroptosis via the STING/NF-κB/p65 pathway. Int Immunopharmacol, 124, 111010. https://doi.org/10.1016/j.intimp.2023.111010
[102] Rizvi F, Lee YR, Diaz-Aragon R, Bawa PS, So J, Florentino RM, et al. (2023). VEGFA mRNA-LNP promotes biliary epithelial cell-to-hepatocyte conversion in acute and chronic liver diseases and reverses steatosis and fibrosis. Cell Stem Cell, 30(12), 1640-1657. e1648. https://doi.org/10.1016/j.stem.2023.10.008
[103] McCaffary D. (2017). STING signalling: an emerging common pathway in autoimmunity and cancer. Immunopharmacol Immunotoxicol, 39(5), 253-258. https://doi.org/10.1080/08923973.2017.1350704
[104] Tankov S, Petrovic M, Lecoultre M, Espinoza F, El-Harane N, Bes V, et al. (2024). Hypoxic glioblastoma-cell-derived extracellular vesicles impair cGAS-STING activity in macrophages. Cell Commun Signal, 22(1), 144. https://doi.org/10.1186/s12964-024-01523-y
[105] Abdullah A, Zhang M, Frugier T, Bedoui S, Taylor JM, & Crack P. (2018). STING-mediated type-I interferons contribute to the neuroinflammatory process and detrimental effects following traumatic brain injury. J Neuroinflammation, 15(1), 323.
[106] Wang YY, Shen D, Zhao LJ, Zeng N, & Hu TH. (2019). Sting is a critical regulator of spinal cord injury by regulating microglial inflammation via interacting with TBK1 in mice. Biochem Biophys Res Commun, 517(4), 741-748. https://doi.org/10.1016/j.bbrc.2019.07.125
[107] Chen K, Lai C, Su Y, Bao WD, Yang LN, Xu P-P, et al. (2022). cGAS-STING-mediated IFN-I response in host defense and neuroinflammatory diseases. Curr Neuropharmacol, 20(2), 362-371. https://doi.org/10.2174/1570159X19666210924110144
[108] Sibal PA, Matsumura S, Ichinose T, Bustos Villalobos I, Morimoto D, Eissa IR, et al. (2024). STING activator 2′ 3′‐cGAMP enhanced HSV‐1‐based oncolytic viral therapy. Mol Oncol, 18(5), 1259-1277. https://doi.org/10.1002/1878-0261.13603
[109] Pan X, Zhang W, Guo H, Wang L, Wu H, Ding L, et al. (2023). Strategies involving STING pathway activation for cancer immunotherapy: mechanism and agonists. Biochem Pharmacol, 213, 115596. https://doi.org/10.1016/j.bcp.2023.115596
[110] Dharshika C, Gonzales J, Chow A, Morales‐Soto W, & Gulbransen BD. (2023). Stimulator of interferon genes (STING) expression in the enteric nervous system and contributions of glial STING in disease. Neurogastroenterol Motil, 35(7), e14553. https://doi.org/10.1111/nmo.14553
[111] Balasubramaniam A, & Srinivasan S. (2023). Role of stimulator of interferon genes (STING) in the enteric nervous system in health and disease. Neurogastroenterol Motil, 35(7), e14603. https://doi.org/10.1111/nmo.14603
[112] Woo MS, Mayer C, Binkle Ladisch L, Sonner JK, Rosenkranz SC, Shaposhnykov A, et al. (2024). STING orchestrates the neuronal inflammatory stress response in multiple sclerosis. Cell, 187(15), 4043-4060. e4030. https://doi.org/10.1016/j.cell.2024.05.031
[113] Liang C, Ke Q, Liu Z, Ren J, Zhang W, Hu J, et al. (2022). BMAL1 moonlighting as a gatekeeper for LINE1 repression and cellular senescence in primates. Nucleic Acids Res, 50(6), 3323-3347. https://doi.org/10.1093/nar/gkac146
[114] Herbstein F, Sapochnik M, Attorresi A, Pollak C, Senin S, Gonilski‐Pacin D, et al. (2024). The SASP factor IL‐6 sustains cell‐autonomous senescent cells via a cGAS‐STING‐NFκB intracrine senescent noncanonical pathway. Aging Cell, 23(10), e14258. https://doi.org/10.1111/acel.14258
[115] Berger G, Knelson EH, Jimenez Macias JL, Nowicki MO, Han S, Panagioti E, et al. (2022). STING activation promotes robust immune response and NK cell–mediated tumor regression in glioblastoma models. Proc Natl Acad Sci U S A, 119(28), e2111003119. https://doi.org/10.1073/pnas.2111003119
[116] Low JT, Brown MC, Reitman ZJ, Bernstock JD, Markert JM, Friedman GK, et al. (2024). Understanding and therapeutically exploiting cGAS/STING signaling in glioblastoma. J Clin Invest, 134(2), e163452. https://doi.org/10.1172/JCI163452
[117] Zhong M, Long M, Han C, Ji S, & Yang Q. (2024). STING is significantly increased in high-grade glioma with high risk of recurrence. Oncoimmunology, 13(1), 2327682. https://doi.org/10.1080/2162402X.2024.2327682
[118] Taniguchi H, Chakraborty S, Takahashi N, Banerjee A, Caeser R, Zhan YA, et al. (2024). ATR inhibition activates cancer cell cGAS/STING-interferon signaling and promotes antitumor immunity in small-cell lung cancer. Sci Adv, 10(39), eado4618. https://doi.org/10.1126/sciadv.ado4618
[119] Song M, Ren J, Zhu Z, Yi Z, Wang C, Liang L, et al. (2025). The STING Signaling: A Novel Target for Central Nervous System Diseases. Cell Mol Neurobiol, 45(1), 33. https://doi.org/10.1007/s10571-025-01550-4
[120] Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 74(3), 229-263. https://doi.org/10.3322/caac.21834
[121] Lei C, Sun W, Wang K, Weng R, Kan X, & Li R. (2025). Artificial intelligence-assisted diagnosis of early gastric cancer: present practice and future prospects. Ann Med, 57(1), 2461679. https://doi.org/10.1080/07853890.2025.2461679
[122] Chen C, & Xu P. (2023). Cellular functions of cGAS-STING signaling. Trends Cell Biol, 33(8), 630-648.
[123] Jneid B, Bochnakian A, Hoffmann C, Delisle F, Djacoto E, Sirven P, et al. (2023). Selective STING stimulation in dendritic cells primes antitumor T cell responses. Sci Immunol, 8(79), eabn6612. https://doi.org/10.1126/sciimmunol.abn6612
[124] Li W, Lu L, Lu J, Wang X, Yang C, Jin J, et al. (2020). cGAS-STING–mediated DNA sensing maintains CD8+ T cell stemness and promotes antitumor T cell therapy. Sci Transl Med, 12(549), eaay9013. https://doi.org/10.1126/scitranslmed.aay9013
[125] Song H, Chen L, Pan X, Shen Y, Ye M, Wang G, et al. (2025). Targeting tumor monocyte-intrinsic PD-L1 by rewiring STING signaling and enhancing STING agonist therapy. Cancer Cell, 43(3), 503-518. e510. https://doi.org/10.1016/j.ccell.2025.02.014
[126] Kaneta A, Nakajima S, Okayama H, Matsumoto T, Saito K, Kikuchi T, et al. (2022). Role of the cGAS-STING pathway in regulating the tumor-immune microenvironment in dMMR/MSI colorectal cancer. Cancer Immunol Immunother, 71(11), 2765-2776. https://doi.org/10.1007/s00262-022-03200-w
[127] Kanoda R, Nakajima S, Fukai S, Saito M, Saito K, Suzuki H, et al. (2024). High levels of tumor cell-intrinsic STING signaling are associated with increased infiltration of CD8+ T cells in dMMR/MSI-H gastric cancer. Sci Rep, 14(1), 20859. https://doi.org/10.1038/s41598-024-71974-3
[128] Marletta S, Caliò A, Bogina G, Rizzo M, Brunelli M, Pedron S, et al. (2023). STING is a prognostic factor related to tumor necrosis, sarcomatoid dedifferentiation, and distant metastasis in clear cell renal cell carcinoma. Virchows Arch, 483(1), 87-96. https://doi.org/10.1007/s00428-023-03549-y
[129] Mekers VE, Kho VM, Ansems M, & Adema GJ. (2022). cGAS/cGAMP/STING signal propagation in the tumor microenvironment: key role for myeloid cells in antitumor immunity. Radiother Oncol, 174, 158-167. https://doi.org/10.1016/j.radonc.2022.07.014
[130] Tan J, Egelston CA, Guo W, Stark JM, & Lee PP. (2024). STING signalling compensates for low tumour mutation burden to drive anti-tumour immunity. EBioMedicine, 101, 105035. https://doi.org/10.1016/j.ebiom.2024.105035
[131] Hu Z, Yu X, Ding R, Liu B, Gu C, Pan XW, et al. (2023). Glycolysis drives STING signaling to facilitate dendritic cell antitumor function. J Clin Invest, 133(7), e166031. https://doi.org/10.1172/JCI166031
[132] Li S, Mirlekar B, Johnson BM, Brickey WJ, Wrobel JA, Yang N, et al. (2022). STING-induced regulatory B cells compromise NK function in cancer immunity. Nature, 610(7931), 373-380. https://doi.org/10.1038/s41586-022-05254-3
[133] Huang X, Huo L, Xiao B, Ouyang Y, Chen F, Li J, et al. (2024). Activating STING/TBK1 suppresses tumor growth via degrading HPV16/18 E7 oncoproteins in cervical cancer. Cell Death Differ, 31(1), 78-89. https://doi.org/10.1038/s41418-023-01242-w
[134] Miyagi S, Watanabe T, Hara Y, Arata M, Uddin MK, Mantoku K, et al. (2021). A STING inhibitor suppresses EBV‐induced B cell transformation and lymphomagenesis. Cancer Sci, 112(12), 5088-5099. https://doi.org/10.1111/cas.15152
[135] Wu SY, Xiao Y, Wei JL, Xu XE, Jin X, Hu X, et al. (2021). MYC suppresses STING-dependent innate immunity by transcriptionally upregulating DNMT1 in triple-negative breast cancer. J Immunother Cancer, 9(7), e002528. https://doi.org/10.1136/jitc-2021-002528
[136] Zhang C, Ye S, Ni J, Cai T, Liu Y, Huang D, et al. (2019). STING signaling remodels the tumor microenvironment by antagonizing myeloid-derived suppressor cell expansion. Cell Death Differ, 26(11), 2314-2328. https://doi.org/10.1038/s41418-019-0302-0
[137] Ong LT, Lee WC, Ma S, Oguz G, Niu Z, Bao Y, et al. (2022). IFI16-dependent STING signaling is a crucial regulator of anti-HER2 immune response in HER2+ breast cancer. Proc Natl Acad Sci U S A, 119(31), e2201376119. https://doi.org/10.1073/pnas.2201376119
[138] Lohard S, Bourgeois N, Maillet L, Gautier F, Fétiveau A, Lasla H, et al. (2020). STING-dependent paracriny shapes apoptotic priming of breast tumors in response to anti-mitotic treatment. Nat Commun, 11(1), 259. https://doi.org/10.1038/s41467-019-13689-y
[139] Bruand M, Barras D, Mina M, Ghisoni E, Morotti M, Lanitis E, et al. (2021). Cell-autonomous inflammation of BRCA1-deficient ovarian cancers drives both tumor-intrinsic immunoreactivity and immune resistance via STING. Cell Rep, 36(3). https://doi.org/10.1016/j.celrep.2021.109412
[140] Nickenig M, Mangan MS, Lee HE, Symeonidis K, Henne A, Kaiser R, et al. (2024). Cutting edge: STING induces ACLY activation and metabolic adaptations in human macrophages through TBK1. J Immunol, 212(1), 7-11. https://doi.org/10.4049/jimmunol.2200835
[141] Liu Y, Fei Y, Wang X, Yang B, Li M, & Luo Z. (2023). Biomaterial-enabled therapeutic modulation of cGAS-STING signaling for enhancing antitumor immunity. Mol Ther, 31(7), 1938-1959. https://doi.org/10.1016/j.ymthe.2023.03.026
[142] Guanizo AC, Luong Q, Jayasekara WSN, de Geus ED, Inampudi C, Xue VS, et al. (2024). A STAT3–STING–IFN axis controls the metastatic spread of small cell lung cancer. Nat Immunol, 25(12), 2259-2269. https://doi.org/10.1038/s41590-024-02014-5
[143] Cai H, Yan L, Liu N, Xu M, & Cai H. (2020). IFI16 promotes cervical cancer progression by upregulating PD-L1 in immunomicroenvironment through STING-TBK1-NF-kB pathway. Biomed Pharmacother, 123, 109790. https://doi.org/10.1016/j.biopha.2019.109790
[144] Lohinai Z, Dora D, Caldwell C, Rivard CJ, Suda K, Yu H, et al. (2022). Loss of STING expression is prognostic in non–small cell lung cancer. J Surg Onco, 125(6), 1042-1052. https://doi.org/10.1002/jso.26804
[145] Wang Y, Guo J, Zhang D, Zhang X, Luo K, & Gong Z. (2025). IDH1/MDH1 deacetylation activates the cGAS-STING pathway by promoting NETosis in acute liver failure. Int Immunopharmacol, 158, 114884. https://doi.org/10.1016/j.intimp.2025.114884
[146] Lam KC, Araya RE, Huang A, Chen Q, Di Modica M, Rodrigues RR, et al. (2021). Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell, 184(21), 5338-5356. e5321. https://doi.org/10.1016/j.cell.2021.09.019
[147] Chen Z, Ji W, Feng W, Cui J, Wang Y, Li F, et al. (2024). PTPRT loss enhances anti–PD-1 therapy efficacy by regulation of STING pathway in non–small cell lung cancer. Sci Transl Med, 16(763), eadl3598. https://doi.org/10.1126/scitranslmed.adl3598
[148] Sun MS, Zhang J, Jiang LQ, Pan YX, Tan JY, Yu F, et al. (2018). TMED2 potentiates cellular IFN responses to DNA viruses by reinforcing MITA dimerization and facilitating its trafficking. Cell Rep, 25(11), 3086-3098. e3083. https://doi.org/10.1016/j.celrep.2018.11.048
[149] Yu L, & Liu P. (2024). cGAS/STING signalling pathway in senescence and oncogenesis. Semin Cancer Biol, 106, 87-102. https://doi.org/10.1016/j.semcancer.2024.08.007
[150] Knelson EH, Ivanova EV, Tarannum M, Campisi M, Lizotte PH, Booker MA, et al. (2022). Activation of tumor-cell STING primes NK-cell therapy. Cancer Immunol Res, 10(8), 947-961. https://doi.org/10.1158/2326-6066.CIR-22-0017
[151] Kumar V, Bauer C, & Stewart IV JH. (2023). Targeting cGAS/STING signaling-mediated myeloid immune cell dysfunction in TIME. J Biomed Sci, 30(1), 48. https://doi.org/10.1186/s12929-023-00942-2
[152] Kho VM, Mekers VE, Span PN, Bussink J, & Adema G. (2021). Radiotherapy and cGAS/STING signaling: impact on MDSCs in the tumor microenvironment. Cell Immunol, 362, 104298. https://doi.org/10.1016/j.cellimm.2021.104298
[153] Lai P, Liu L, Bancaro N, Troiani M, Calì B, Li Y, et al. (2025). Mitochondrial DNA released by senescent tumor cells enhances PMN-MDSC-driven immunosuppression through the cGAS-STING pathway. Immunity, 58(4), 811-825. e817. https://doi.org/10.1016/j.immuni.2025.03.005
[154] Chen L, Alabdullah M, & Mahnke K. (2023). Adenosine, bridging chronic inflammation and tumor growth. Front Immunol, 14, 1258637. https://doi.org/10.3389/fimmu.2023.1258637
[155] Keramati F, Leijte GP, Bruse N, Grondman I, Habibi E, Ruiz-Moreno C, et al. (2025). Systemic inflammation impairs myelopoiesis and interferon type I responses in humans. Nat Immunol, 26(5), 737-747. https://doi.org/10.1038/s41590-025-02136-4
[156] Vasiyani H, Wadhwa B, & Singh R. (2023). Regulation of cGAS-STING signalling in cancer: Approach for combination therapy. Biochim Biophys Acta Rev Cancer, 1878(3), 188896. https://doi.org/10.1016/j.bbcan.2023.188896
[157] Lin H, Xie Y, Kong Y, Yang L, & Li M. (2021). Identification of molecular subtypes and prognostic signature for hepatocellular carcinoma based on genes associated with homologous recombination deficiency. Sci Rep, 11(1), 24022. https://doi.org/10.1038/s41598-021-03432-3
Type
Published
Data Availability Statement
Not Applicable
Issue
Section
License
Copyright (c) 2025 Life Conflux

This work is licensed under a Creative Commons Attribution 4.0 International License.







