Exploring Long Noncoding RNAs and Ferroptosis in Cancer Progression
DOI:
https://doi.org/10.71321/jjrzya36Keywords:
Ferroptosis; Long non-coding RNAs; OncotherapyAbstract
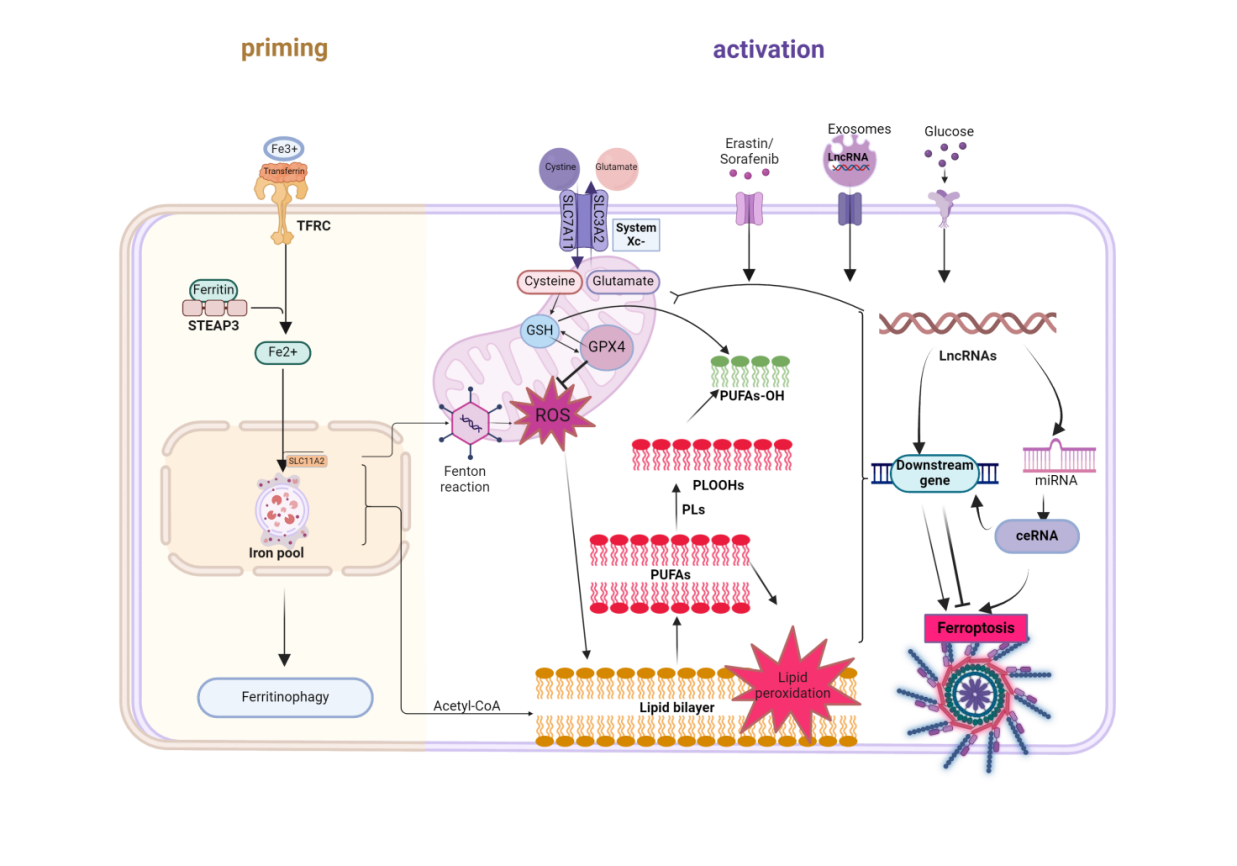
Ferroptosis is a new type of regulated cell death produced by iron-dependent accumulation of reactive oxygen species in lipids, which is different from apoptosis, pyroptosis and autophagy, and plays the role of a "double-edged sword" in tumor therapy. Ferroptosis-related lncRNA are involved in tumor cell death by regulating lipid metabolism and ferroptosis-associated genes, thus becoming one of the hotspots in the field of tumor-targeted therapy. In this paper, we review the role of ferroptosis and its related lncRNA in inhibiting the development of tumor cells and improving the therapeutic effect of drugs, which is expected to provide a new strategy for the treatment of tumors.
References
[1] DIXON SJ, LEMBERG KM, LAMPRECHT MR, SKOUTA R, ZAITSEV EM, GLEASON CE, et al. (2012).Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell, 149(5): 1060-1072. https://doi.org/10.1016/j.cell.2012.03.042
[2] ELMORE S. (2007).Apoptosis: a review of programmed cell death. Toxicol Pathol, 35(4): 495-516. https://doi.org/10.1080/01926230701320337
[3] DU T, GAO J, LI P, WANG Y, QI Q, LIU X, et al. (2021).Pyroptosis, metabolism, and tumor immune microenvironment. Clin Transl Med, 11(8): e492. https://doi.org/10.1002/ctm2.492
[4] CAO JY, DIXON SJ. (2016).Mechanisms of ferroptosis. Cell Mol Life Sci, 73(11-12): 2195-2209. https://doi.org/10.1007/s00018-016-2194-1
[5] MOU Y, WANG J, WU J, HE D, ZHANG C, DUAN C, et al. (2019).Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol, 12(1): 34. https://doi.org/10.1186/s13045-019-0720-y
[6] XIE Y, HOU W, SONG X, YU Y, HUANG J, SUN X, et al. (2016).Ferroptosis: process and function. Cell Death Differ, 23(3): 369-379. https://doi.org/10.1038/cdd.2015.158
[7] CESANA M, CACCHIARELLI D, LEGNINI I, SANTINI T, STHANDIER O, CHINAPPI M, et al. (2011).A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell, 147(2): 358-369. https://doi.org/10.1016/j.cell.2011.09.028
[8] STATELLO L, GUO CJ, CHEN LL, HUARTE M. (2021).Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol, 22(2): 96-118. https://doi.org/10.1038/s41580-020-00315-9
[9] HUANG J, WANG J, HE H, HUANG Z, WU S, CHEN C, et al. (2021).Close interactions between lncRNAs, lipid metabolism and ferroptosis in cancer. Int J Biol Sci, 17(15): 4493-4513. https://doi.org/10.7150/ijbs.66181
[10] LIN W, ZHOU Q, WANG CQ, ZHU L, BI C, ZHANG S, et al. (2020).LncRNAs regulate metabolism in cancer. Int J Biol Sci, 16(7): 1194-1206. https://doi.org/10.7150/ijbs.40769
[11] LI J, MENG H, BAI Y, WANG K. (2016).Regulation of lncRNA and its role in cancer metastasis. Oncol Res, 23(5): 205-217. https://doi.org/10.3727/096504016x14549667334007
[12] XU W, ZHOU G, WANG H, LIU Y, CHEN B, CHEN W, et al. (2020).Circulating lncRNA SNHG11 as a novel biomarker for early diagnosis and prognosis of colorectal cancer. Int J Cancer, 146(10): 2901-2912. https://doi.org/10.1002/ijc.32747
[13] JIANG X, STOCKWELL BR, CONRAD M. (2021).Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol, 22(4): 266-282. https://doi.org/10.1038/s41580-020-00324-8
[14] VASAN N, BASELGA J, HYMAN DM. (2019).A view on drug resistance in cancer. Nature, 575(7782): 299-309. https://doi.org/10.1038/s41586-019-1730-1
[15] AVERETT C, BHARDWAJ A, ARORA S, SRIVASTAVA SK, KHAN MA, AHMAD A, et al. (2016).Honokiol suppresses pancreatic tumor growth, metastasis and desmoplasia by interfering with tumor-stromal cross-talk. Carcinogenesis, 37(11): 1052-1061. https://doi.org/10.1093/carcin/bgw096
[16] TANG D, KROEMER G. (2020).Ferroptosis. Curr Biol, 30(21): R1292-r1297. https://doi.org/10.1016/j.cub.2020.09.068
[17] GRAHAM RM, CHUA AC, HERBISON CE, OLYNYK JK, TRINDER D. (2007).Liver iron transport. World J Gastroenterol, 13(35): 4725-4736. https://doi.org/10.3748/wjg.v13.i35.4725
[18] HE YJ, LIU XY, XING L, WAN X, CHANG X, JIANG HL. (2020).Fenton reaction-independent ferroptosis therapy via glutathione and iron redox couple sequentially triggered lipid peroxide generator. Biomaterials, 241: 119911. https://doi.org/10.1016/j.biomaterials.2020.119911
[19] CHENG Z, LI Y. (2007).What is responsible for the initiating chemistry of iron-mediated lipid peroxidation: an update. Chem Rev, 107(3): 748-766. https://doi.org/10.1021/cr040077w
[20] BAO X, LUO X, BAI X, LV Y, WENG X, ZHANG S, et al. (2023).Cigarette tar mediates macrophage ferroptosis in atherosclerosis through the hepcidin/FPN/SLC7A11 signaling pathway. Free Radic Biol Med, 201: 76-88. https://doi.org/10.1016/j.freeradbiomed.2023.03.006
[21] WILBON AS, SHEN J, RUCHALA P, ZHOU M, PAN Y. (2023).Structural basis of ferroportin inhibition by minihepcidin PR73. PLoS Biol, 21(1): e3001936. https://doi.org/10.1371/journal.pbio.3001936
[22] CARDONA CJ, MONTGOMERY MR. (2023).Iron regulatory proteins: players or pawns in ferroptosis and cancer? Front Mol Biosci, 10: 1229710. https://doi.org/10.3389/fmolb.2023.1229710
[23] VON KRUSENSTIERN AN, ROBSON RN, QIAN N, QIU B, HU F, REZNIK E, et al. (2023).Identification of essential sites of lipid peroxidation in ferroptosis. Nat Chem Biol, 19(6): 719-730. https://doi.org/10.1038/s41589-022-01249-3
[24] LIANG D, MINIKES AM, JIANG X. (2022).Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell, 82(12): 2215-2227. https://doi.org/10.1016/j.molcel.2022.03.022
[25] YUAN ZH, LIU T, WANG H, XUE LX, WANG JJ. (2021).Fatty Acids Metabolism: The Bridge Between Ferroptosis and Ionizing Radiation. Front Cell Dev Biol, 9: 675617. https://doi.org/10.3389/fcell.2021.675617
[26] YANG WS, STOCKWELL BR. (2016).Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol, 26(3): 165-176. https://doi.org/10.1016/j.tcb.2015.10.014
[27] DIXON SJ, STOCKWELL BR. (2014).The role of iron and reactive oxygen species in cell death. Nat Chem Biol, 10(1): 9-17. https://doi.org/10.1038/nchembio.1416
[28] CHEN X, LI J, KANG R, KLIONSKY DJ, TANG D. (2021).Ferroptosis: machinery and regulation. Autophagy, 17(9): 2054-2081. https://doi.org/10.1080/15548627.2020.1810918
[29] DE BAAT A, MEIER DT, FONTANA A, BöNI-SCHNETZLER M, DONATH MY. (2023).Cystine/Glutamate antiporter system xc- deficiency impairs macrophage glutathione metabolism and cytokine production. PLoS One, 18(10): e0291950. https://doi.org/10.1371/journal.pone.0291950
[30] ZHAO Y, LI Y, ZHANG R, WANG F, WANG T, JIAO Y. (2020).The role of erastin in ferroptosis and its prospects in cancer therapy. Onco Targets Ther, 13: 5429-5441. https://doi.org/10.2147/ott.S254995
[31] YAN Y, TENG H, HANG Q, KONDIPARTHI L, LEI G, HORBATH A, et al. (2023).SLC7A11 expression level dictates differential responses to oxidative stress in cancer cells. Nat Commun, 14(1): 3673. https://doi.org/10.1038/s41467-023-39401-9
[32] KOPPULA P, ZHUANG L, GAN B. (2021).Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell, 12(8): 599-620. https://doi.org/10.1007/s13238-020-00789-5
[33] XU T, DING W, JI X, AO X, LIU Y, YU W, et al. (2019).Molecular mechanisms of ferroptosis and its role in cancer therapy. J Cell Mol Med, 23(8): 4900-4912. https://doi.org/10.1111/jcmm.14511
[34] LATUNDE-DADA GO. (2017).Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj, 1861(8): 1893-1900. https://doi.org/10.1016/j.bbagen.2017.05.019
[35] URSINI F, MAIORINO M. (2020).Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic Biol Med, 152: 175-185. https://doi.org/10.1016/j.freeradbiomed.2020.02.027
[36] SEILER A, SCHNEIDER M, FöRSTER H, ROTH S, WIRTH EK, CULMSEE C, et al. (2008).Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab, 8(3): 237-248. https://doi.org/10.1016/j.cmet.2008.07.005
[37] THéRY C, ZITVOGEL L, AMIGORENA S. (2002).Exosomes: composition, biogenesis and function. Nat Rev Immunol, 2(8): 569-579. https://doi.org/10.1038/nri855
[38] BROWN CW, MERCURIO AM. (2020).Ferroptosis resistance mediated by exosomal release of iron. Mol Cell Oncol, 7(3): 1730144. https://doi.org/10.1080/23723556.2020.1730144
[39] SUNG H, FERLAY J, SIEGEL RL, LAVERSANNE M, SOERJOMATARAM I, JEMAL A, et al. (2021).Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin, 71(3): 209-249. https://doi.org/10.3322/caac.21660
[40] NIE J, LIN B, ZHOU M, WU L, ZHENG T. (2018).Role of ferroptosis in hepatocellular carcinoma. J Cancer Res Clin Oncol, 144(12): 2329-2337. https://doi.org/10.1007/s00432-018-2740-3
[41] CAPELLETTI MM, MANCEAU H, PUY H, PEOC'H K. (2020).Ferroptosis in liver diseases: an overview. Int J Mol Sci, 21(14): 4908. https://doi.org/10.3390/ijms21144908
[42] QIN Y, ZHANG D, ZHANG H, HOU L, WANG Z, YANG L, et al. (2022).Construction of a ferroptosis-related five-lncRNA signature for predicting prognosis and immune response in thyroid carcinoma. Cancer Cell Int, 22(1): 296. https://doi.org/10.1186/s12935-022-02674-z
[43] FEI X, HU C, WANG X, LU C, CHEN H, SUN B, et al. (2021).Construction of a Ferroptosis-Related Long Non-coding RNA Prognostic Signature and Competing Endogenous RNA Network in Lung Adenocarcinoma. Front Cell Dev Biol, 9: 751490. https://doi.org/10.3389/fcell.2021.751490
[44] WU L, PAN C, WEI X, SHI Y, ZHENG J, LIN X, et al. (2018).lncRNA KRAL reverses 5-fluorouracil resistance in hepatocellular carcinoma cells by acting as a ceRNA against miR-141. Cell Commun Signal, 16(1): 47. https://doi.org/10.1186/s12964-018-0260-z
[45] NGUYEN T, NIOI P, PICKETT CB. (2009).The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem, 284(20): 13291-13295. https://doi.org/10.1074/jbc.R900010200
[46] SUN X, OU Z, CHEN R, NIU X, CHEN D, KANG R, et al. (2016).Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology, 63(1): 173-184. https://doi.org/10.1002/hep.28251
[47] KANSANEN E, KUOSMANEN SM, LEINONEN H, LEVONEN AL. (2013).The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol, 1(1): 45-49. https://doi.org/10.1016/j.redox.2012.10.001
[48] DENICOLA GM, KARRETH FA, HUMPTON TJ, GOPINATHAN A, WEI C, FRESE K, et al. (2011).Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature, 475(7354): 106-109. https://doi.org/10.1038/nature10189
[49] QI W, LI Z, XIA L, DAI J, ZHANG Q, WU C, et al. (2019).LncRNA GABPB1-AS1 and GABPB1 regulate oxidative stress during erastin-induced ferroptosis in HepG2 hepatocellular carcinoma cells. Sci Rep, 9(1): 16185. https://doi.org/10.1038/s41598-019-52837-8
[50] HE GN, BAO NR, WANG S, XI M, ZHANG TH, CHEN FS. (2021).Ketamine Induces ferroptosis of liver cancer cells by targeting lncRNA PVT1/miR-214-3p/GPX4. Drug Des Devel Ther, 15: 3965-3978. https://doi.org/10.2147/dddt.S332847
[51] KANG X, HUO Y, JIA S, HE F, LI H, ZHOU Q, et al. (2022).Silenced LINC01134 enhances oxaliplatin sensitivity by facilitating ferroptosis through GPX4 in hepatocarcinoma. Front Oncol, 12: 939605. https://doi.org/10.3389/fonc.2022.939605
[52] ZHANG Y, LUO M, CUI X, O'CONNELL D, YANG Y. (2022).Long noncoding RNA NEAT1 promotes ferroptosis by modulating the miR-362-3p/MIOX axis as a ceRNA. Cell Death Differ, 29(9): 1850-1863. https://doi.org/10.1038/s41418-022-00970-9
[53] ALDUAIS Y, ZHANG H, FAN F, CHEN J, CHEN B. (2023).Non-small cell lung cancer (NSCLC): A review of risk factors, diagnosis, and treatment. Medicine (Baltimore), 102(8): e32899. https://doi.org/10.1097/md.0000000000032899
[54] RAMALINGAM SS, VANSTEENKISTE J, PLANCHARD D, CHO BC, GRAY JE, OHE Y, et al. (2020).Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med, 382(1): 41-50. https://doi.org/10.1056/NEJMoa1913662
[55] WANG M, HERBST RS, BOSHOFF C. (2021).Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med, 27(8): 1345-1356. https://doi.org/10.1038/s41591-021-01450-2
[56] KANG R, KROEMER G, TANG D. (2019).The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med, 133: 162-168. https://doi.org/10.1016/j.freeradbiomed.2018.05.074
[57] MAO C, WANG X, LIU Y, WANG M, YAN B, JIANG Y, et al. (2018).A G3BP1-interacting lncRNA promotes ferroptosis and apoptosis in cancer via nuclear sequestration of p53. Cancer Res, 78(13): 3484-3496. https://doi.org/10.1158/0008-5472.Can-17-3454
[58] ZHANG EB, YIN DD, SUN M, KONG R, LIU XH, YOU LH, et al. (2014).P53-regulated long non-coding RNA TUG1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death Dis, 5(5): e1243. https://doi.org/10.1038/cddis.2014.201
[59] LU KH, LI W, LIU XH, SUN M, ZHANG ML, WU WQ, et al. (2013).Long non-coding RNA MEG3 inhibits NSCLC cells proliferation and induces apoptosis by affecting p53 expression. BMC Cancer, 13: 461. https://doi.org/10.1186/1471-2407-13-461
[60] XU J, SU C, ZHAO F, TAO J, HU D, SHI A, et al. (2018).Paclitaxel promotes lung cancer cell apoptosis via MEG3-P53 pathway activation. Biochem Biophys Res Commun, 504(1): 123-128. https://doi.org/10.1016/j.bbrc.2018.08.142
[61] HAYANO M, YANG WS, CORN CK, PAGANO NC, STOCKWELL BR. (2016).Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ, 23(2): 270-278. https://doi.org/10.1038/cdd.2015.93
[62] WU H, LIU A. (2021).Long non-coding RNA NEAT1 regulates ferroptosis sensitivity in non-small-cell lung cancer. J Int Med Res, 49(3): 300060521996183. https://doi.org/10.1177/0300060521996183
[63] FU M, GU J, JIANG P, QIAN H, XU W, ZHANG X. (2019).Exosomes in gastric cancer: roles, mechanisms, and applications. Mol Cancer, 18(1): 41. https://doi.org/10.1186/s12943-019-1001-7
[64] ZHANG H, WANG M, HE Y, DENG T, LIU R, WANG W, et al. (2021).Chemotoxicity-induced exosomal lncFERO regulates ferroptosis and stemness in gastric cancer stem cells. Cell Death Dis, 12(12): 1116. https://doi.org/10.1038/s41419-021-04406-z
[65] PéREZ-HERAS AM, MAYNERIS-PERXACHS J, COFáN M, SERRA-MIR M, CASTELLOTE AI, LóPEZ-SABATER C, et al. (2018).Long-chain n-3 PUFA supplied by the usual diet decrease plasma stearoyl-CoA desaturase index in non-hypertriglyceridemic older adults at high vascular risk. Clin Nutr, 37(1): 157-162. https://doi.org/10.1016/j.clnu.2016.11.009
[66] NIU R, ZHAO F, DONG Z, LI Z, LI S. (2022).A stratification system of ferroptosis and iron-metabolism related LncRNAs guides the prediction of the survival of patients with esophageal squamous cell carcinoma. Front Oncol, 12: 1010074. https://doi.org/10.3389/fonc.2022.1010074
[67] CHEN C, ZHAO J, LIU JN, SUN C. (2021).Mechanism and role of the neuropeptide LGI1 receptor ADAM23 in regulating biomarkers of ferroptosis and progression of esophageal cancer. Dis Markers, 2021: 9227897. https://doi.org/10.1155/2021/9227897
[68] YANG H, HU Y, WENG M, LIU X, WAN P, HU Y, et al. (2022).Hypoxia inducible lncRNA-CBSLR modulates ferroptosis through m6A-YTHDF2-dependent modulation of CBS in gastric cancer. J Adv Res, 37: 91-106. https://doi.org/10.1016/j.jare.2021.10.001
[69] YAO X, YANG P, JIN Z, JIANG Q, GUO R, XIE R, et al. (2019).Multifunctional nanoplatform for photoacoustic imaging-guided combined therapy enhanced by CO induced ferroptosis. Biomaterials, 197: 268-283. https://doi.org/10.1016/j.biomaterials.2019.01.026
[70] HUANG G, XIANG Z, WU H, HE Q, DOU R, LIN Z, et al. (2022).The lncRNA BDNF-AS/WDR5/FBXW7 axis mediates ferroptosis in gastric cancer peritoneal metastasis by regulating VDAC3 ubiquitination. Int J Biol Sci, 18(4): 1415-1433. https://doi.org/10.7150/ijbs.69454
[71] MALDONADO EN, SHELDON KL, DEHART DN, PATNAIK J, MANEVICH Y, TOWNSEND DM, et al. (2013).Voltage-dependent anion channels modulate mitochondrial metabolism in cancer cells: regulation by free tubulin and erastin. J Biol Chem, 288(17): 11920-11929. https://doi.org/10.1074/jbc.M112.433847
[72] PANG JY, LI PL, AFERIN B, YAN Y, WANG JM. (2021). New strategies for cancer treatment based on ferroptosis. Modern Oncology, 29(06): 1058-1061.
[73] BRAY F, LAVERSANNE M, SUNG H, FERLAY J, SIEGEL RL, SOERJOMATARAM I, et al. (2024).Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 74(3): 229-263. https://doi.org/10.3322/caac.21834
[74] CASADEI C, DIZMAN N, SCHEPISI G, CURSANO MC, BASSO U, SANTINI D, et al. (2019).Targeted therapies for advanced bladder cancer: new strategies with FGFR inhibitors. Ther Adv Med Oncol, 11: 1758835919890285. https://doi.org/10.1177/1758835919890285
[75] HERNANDO POLO S, MORENO MUñOZ D, ROSERO RODRíGUEZ AC, SILVA RUIZ J, ROSERO RODRíGUEZ DI, COUñAGO F. (2021).Changing the History of Prostate Cancer with New Targeted Therapies. Biomedicines, 9(4):392. https://doi.org/10.3390/biomedicines9040392
[76] POSADAS EM, LIMVORASAK S, FIGLIN RA. (2017).Targeted therapies for renal cell carcinoma. Nat Rev Nephrol, 13(8): 496-511. https://doi.org/10.1038/nrneph.2017.82
[77] ZHANG Y, GUO S, WANG S, LI X, HOU D, LI H, et al. (2021).LncRNA OIP5-AS1 inhibits ferroptosis in prostate cancer with long-term cadmium exposure through miR-128-3p/SLC7A11 signaling. Ecotoxicol Environ Saf, 220: 112376. https://doi.org/10.1016/j.ecoenv.2021.112376
[78] LI HJ, LI X, PANG H, PAN JJ, XIE XJ, CHEN W. (2015).Long non-coding RNA UCA1 promotes glutamine metabolism by targeting miR-16 in human bladder cancer. Jpn J Clin Oncol, 45(11): 1055-1063. https://doi.org/10.1093/jjco/hyv132
[79] BUCZKOWSKA J, SZELIGA M. (2023).Two Faces of Glutaminase GLS2 in Carcinogenesis. Cancers (Basel), 15(23). https://doi.org/10.3390/cancers15235566
[80] JIAO K, CHENG J, WANG Q, HAO M. (2024).LncRNA UCA1 enhances NRF2 expression through the m(6)A pathway to mitigate oxidative stress and ferroptosis in aging cardiomyocytes. J Bioenerg Biomembr, 56(6): 607-617. https://doi.org/10.1007/s10863-024-10045-8
[81] LUO W, WANG J, XU W, MA C, WAN F, HUANG Y, et al. (2021).LncRNA RP11-89 facilitates tumorigenesis and ferroptosis resistance through PROM2-activated iron export by sponging miR-129-5p in bladder cancer. Cell Death Dis, 12(11): 1043. https://doi.org/10.1038/s41419-021-04296-1
[82] BROWN CW, AMANTE JJ, CHHOY P, ELAIMY AL, LIU H, ZHU LJ, et al. (2019).Prominin2 Drives Ferroptosis Resistance by Stimulating Iron Export. Dev Cell, 51(5): 575-586.e574. https://doi.org/10.1016/j.devcel.2019.10.007
[83] CORADDUZZA D, CONGIARGIU A, CHEN Z, ZINELLU A, CARRU C, MEDICI S. (2023).Ferroptosis and Senescence: A Systematic Review. Int J Mol Sci, 24(4). https://doi.org/10.3390/ijms24043658
[84] MAZHAR M, DIN AU, ALI H, YANG G, REN W, WANG L, et al. (2021).Implication of ferroptosis in aging. Cell Death Discov, 7(1): 149. https://doi.org/10.1038/s41420-021-00553-6
[85] GONG D, CHEN M, WANG Y, SHI J, HOU Y. (2022).Role of ferroptosis on tumor progression and immunotherapy. Cell Death Discov, 8(1): 427. https://doi.org/10.1038/s41420-022-01218-8
[86] LU Y, XIE X, LUO L. (2024).Ferroptosis crosstalk in anti-tumor immunotherapy: molecular mechanisms, tumor microenvironment, application prospects. Apoptosis, 29(11-12): 1914-1943. https://doi.org/10.1007/s10495-024-01997-8
[87] LI Q, CHEN K, ZHANG T, JIANG D, CHEN L, JIANG J, et al. (2023).Understanding sorafenib-induced ferroptosis and resistance mechanisms: Implications for cancer therapy. Eur J Pharmacol, 955: 175913. https://doi.org/10.1016/j.ejphar.2023.175913
[88] PEARSON H, MARSHALL LV, CARCELLER F. (2020).Sorafenib in pediatric hepatocellular carcinoma from a clinician perspective. Pediatr Hematol Oncol, 37(5): 412-423. https://doi.org/10.1080/08880018.2020.1740844
[89] LADD AD, DUARTE S, SAHIN I, ZARRINPAR A. (2024).Mechanisms of drug resistance in HCC. Hepatology, 79(4): 926-940. https://doi.org/10.1097/hep.0000000000000237
Type
Published
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
Issue
Section
License
Copyright (c) 2025 Life Conflux

This work is licensed under a Creative Commons Attribution 4.0 International License.







