Genetic Risk Mapping and Insights into Esophageal Adenocarcinoma Development
DOI:
https://doi.org/10.71321/mczdpt96Keywords:
Esophageal adenocarcinoma, Mendelian randomization, single-nucleotide polymorphisms, risk factor, early detectionAbstract
Background: Esophageal adenocarcinoma (EAC) is a malignant tumor that has been increasing in incidence over the past few decades. Identifying the risk factors associated with EAC is crucial for prevention and early detection strategies.
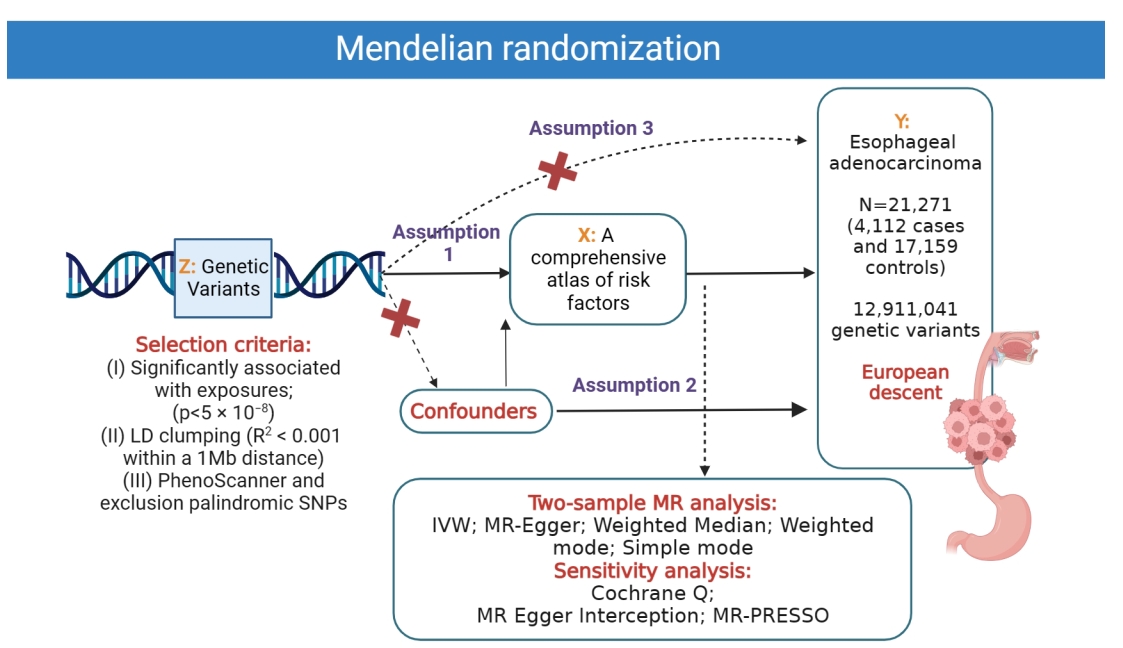
Methods: This study aimed to investigate the potential risk factors for EAC using a Mendelian randomization (MR) approach. We attained genetic variants associated with 52 exposure factors s from available large-scale genome-wide association studies.
Results: Genetic predisposition to childhood body mass index, forced expiratory volume in 1-second, glomerular filtration rate, telomere length, alcohol consumption, anxiety or depression, smoking, coffee consumption, time spent watching television, basal metabolic rate, body fat percentage, body mass index, hip circumference, obesity class 1, obesity class 2, trunk fat percentage, waist circumference, waist-to-hip ratio, iron, Barrett's esophagus, and gastroesophageal reflux disease were associated with increased risk of EAC. In addition, we demonstrated that age at first birth, age at first sexual intercourse, age at menarche, years of schooling, type 1 diabetes, and fruit intake could suggestively decrease the odds of EAC.
Conclusion: This study represents the inaugural MR investigation to present a comprehensive atlas of risk factors associated with EAC, which will aid in advancing the formulation of efficacious preventive and early detection approaches.
References
[1] Avgerinos, K. I., Spyrou, N., Mantzoros, C. S., & Dalamaga, M. (2019, Mar). Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism, 92, 121-135. https://doi.org/10.1016/j.metabol.2018.11.001
[2] Burgess, S., & Labrecque, J. (2018). Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. European journal of epidemiology, 33(10), 947-952. https://doi.org/10.1007/s10654-018-0424-6
[3] Chen, L., Lu, H., Peng, D., Cao, L. L., Ballout, F., Srirmajayam, K., et al. (2023, Mar). Activation of NOTCH signaling via DLL1 is mediated by APE1-redox-dependent NF-κB activation in oesophageal adenocarcinoma. Gut, 72(3), 421-432. https://doi.org/10.1136/gutjnl-2022-327076
[4] Coleman, H. G., Xie, S.-H., & Lagergren, J. (2018, 2018/1//). The Epidemiology of Esophageal Adenocarcinoma. Gastroenterology, 154(2), 390-405. https://doi.org/10.1053/j.gastro.2017.07.046
[5] Global Burden of Disease Cancer, C., Kocarnik, J. M., Compton, K., Dean, F. E., Fu, W., Gaw, B. L., et al. (2022, 2022/3/1/). Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA oncology, 8(3), 420-444. https://doi.org/10.1001/jamaoncol.2021.6987
[6] Innes, H., Nischalke, H. D., Guha, I. N., Weiss, K. H., Irving, W., Gotthardt, D., et al. (2022, May). The rs429358 Locus in Apolipoprotein E Is Associated With Hepatocellular Carcinoma in Patients With Cirrhosis. Hepatol Commun, 6(5), 1213-1226. https://doi.org/10.1002/hep4.1886
[7] Joseph, A., Raja, S., Kamath, S., Jang, S., Allende, D., McNamara, M., et al. (2022, May 2). Esophageal adenocarcinoma: A dire need for early detection and treatment. Cleve Clin J Med, 89(5), 269-279. https://doi.org/10.3949/ccjm.89a.21053
[8] Kashif, M., Yao, H., Schmidt, S., Chen, X., Truong, M., Tüksammel, E., et al. (2023, Apr). ROS-lowering doses of vitamins C and A accelerate malignant melanoma metastasis. Redox Biol, 60, 102619. https://doi.org/10.1016/j.redox.2023.102619
[9] Kawabata, T. (2022, Jul 8). Iron-Induced Oxidative Stress in Human Diseases. Cells, 11(14). https://doi.org/10.3390/cells11142152
[10] Lega, I. C., & Lipscombe, L. L. (2020, Feb 1). Review: Diabetes, Obesity, and Cancer-Pathophysiology and Clinical Implications. Endocr Rev, 41(1). https://doi.org/10.1210/endrev/bnz014
[11] Li, W., Wang, R., & Wang, W. (2022). Exploring the causality and pathogenesis of systemic lupus erythematosus in breast cancer based on Mendelian randomization and transcriptome data analyses. Front Immunol, 13, 1029884. https://doi.org/10.3389/fimmu.2022.1029884
[12] Mei, H., Yin, B., Yang, W., Zhang, J., Lu, H., Qi, X., et al. (2022). Associations between Gene-Gene Interaction and Overweight/Obesity of 12-Month-Old Chinese Infants. Biomed Res Int, 2022, 1499454. https://doi.org/10.1155/2022/1499454
[13] Nøst, T. H., Alcala, K., Urbarova, I., Byrne, K. S., Guida, F., Sandanger, T. M., et al. (2021, Aug). Systemic inflammation markers and cancer incidence in the UK Biobank. Eur J Epidemiol, 36(8), 841-848. https://doi.org/10.1007/s10654-021-00752-6
[14] Peng, H., Wu, X., Wen, Y., Du, X., Li, C., Liang, H., et al. (2021, 2021/4//). Age at first birth and lung cancer: a two-sample Mendelian randomization study. Translational lung cancer research, 10(4), 1720-1733. https://doi.org/10.21037/tlcr-20-1216
[15] Pisanu, C., Williams, M. J., Ciuculete, D. M., Olivo, G., Del Zompo, M., Squassina, A., et al. (2019, Nov 21). Evidence that genes involved in hedgehog signaling are associated with both bipolar disorder and high BMI. Transl Psychiatry, 9(1), 315. https://doi.org/10.1038/s41398-019-0652-x
[16] Qin, M., Shao, B., Lin, L., Zhang, Z. Q., Sheng, Z. G., Qin, L., et al. (2023, Jan). Molecular mechanism of the unusual biphasic effects of the natural compound hinokitiol on iron-induced cellular DNA damage. Free Radic Biol Med, 194, 163-171. https://doi.org/10.1016/j.freeradbiomed.2022.11.042
[17] Rajendra, S., Sharma, P., Gautam, S. D., Saxena, M., Kapur, A., Sharma, P., et al. (2020, Feb 5). Association of Biomarkers for Human Papillomavirus With Survival Among Adults With Barrett High-grade Dysplasia and Esophageal Adenocarcinoma. JAMA Netw Open, 3(2), e1921189. https://doi.org/10.1001/jamanetworkopen.2019.21189
[18] Redston, M., Noffsinger, A., Kim, A., Akarca, F. G., Rara, M., Stapleton, D., et al. (2022, Feb). Abnormal TP53 Predicts Risk of Progression in Patients With Barrett's Esophagus Regardless of a Diagnosis of Dysplasia. Gastroenterology, 162(2), 468-481. https://doi.org/10.1053/j.gastro.2021.10.038
[19] Sekula, P., Del Greco M, F., Pattaro, C., & Köttgen, A. (2016, 2016/11//). Mendelian Randomization as an Approach to Assess Causality Using Observational Data. Journal of the American Society of Nephrology, 27(11), 3253-3265. https://doi.org/10.1681/ASN.2016010098
[20] Siegel, R. L., Miller, K. D., & Jemal, A. (2020, Jan). Cancer statistics, 2020. CA Cancer J Clin, 70(1), 7-30. https://doi.org/10.3322/caac.21590
[21] Su, X., Shen, Z., Yang, Q., Sui, F., Pu, J., Ma, J., et al. (2019). Vitamin C kills thyroid cancer cells through ROS-dependent inhibition of MAPK/ERK and PI3K/AKT pathways via distinct mechanisms. Theranostics, 9(15), 4461-4473. https://doi.org/10.7150/thno.35219
[22] Tanes, C., Bittinger, K., Gao, Y., Friedman, E. S., Nessel, L., Paladhi, U. R., et al. (2021, Mar 10). Role of dietary fiber in the recovery of the human gut microbiome and its metabolome. Cell Host Microbe, 29(3), 394-407.e395. https://doi.org/10.1016/j.chom.2020.12.012
[23] Telomeres Mendelian Randomization, C., Haycock, P. C., Burgess, S., Nounu, A., Zheng, J., Okoli, G. N., et al. (2017, 2017/5/1/). Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA oncology, 3(5), 636-651. https://doi.org/10.1001/jamaoncol.2016.5945
Type
Published
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
Issue
Section
License
Copyright (c) 2025 Life Conflux

This work is licensed under a Creative Commons Attribution 4.0 International License.







