Global research status and hotspots of squamous cell carcinoma endothelial cells in the last 20 years: A bibliometric analysis
DOI:
https://doi.org/10.71321/m8qh8d83Keywords:
Squamous cell carcinoma; Endothelial cells; Research progress; Bibliometric analysis; hotspotAbstract
Objective: The aim is to analyze the current research status and hotspots of squamous cell carcinoma endothelial cells and to provide a reference for the following fundamental research and clinical treatment.
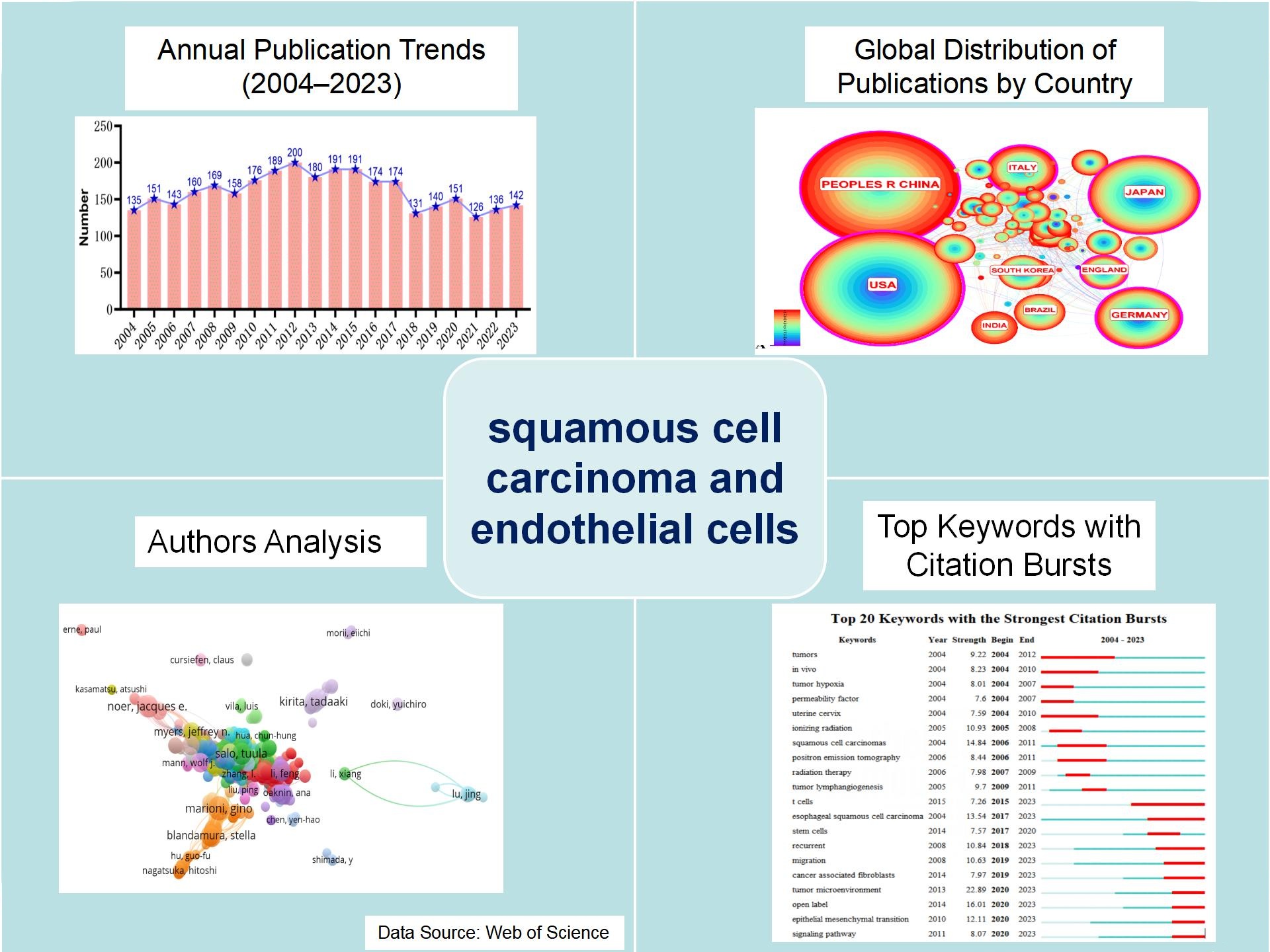
Methods: The Web of Science Core Collection (WOSCC) database was searched for literature on squamous cell carcinoma endothelial cells from January 1, 2004, to December 31, 2023. The results were analyzed for research trends, authors, countries, research institutions, and keywords using CiteSpace, VOSviewer, and the bibliometrixc data package in R language.
Results: 3217 articles were included in the analysis, and the number of articles published in the past five years is relatively stable. The number of publications from 89 countries and 3,163 institutions has been relatively stable in the past five years, of which 800 are from China, 195 are from the University of Texas System, which is higher than that of other countries and institutions, and there is a big difference in the number of publications between countries and institutions. Prof. Marioni Gino has published 16 relevant papers, and 713 citations have been given to Forkman J, so there is more frequent cooperation between scholars. There are more frequent collaborations among scholars. Eight hundred sixty-eight journals published relevant papers, with Oral Oncology having the highest number of articles and Cancer Research having the highest number of citations. The most cited reference is “Hallmarks of cancer: the next generation,” DOI:10.1016/j.cell.2011.02.013 (intensity 21.75). In the last five years, keywords with high intensity are migration, tumor microenvironment, open-label, epithelial-mesenchymal transition, t cells, esophageal squamous cell carcinoma, and recurrent.
Conclusion: The development of squamous cell carcinoma endothelial cell research is uneven among different countries, institutions, and authors, and the journal Oral Oncology publishes the most relevant papers, with current research hotspots including metastasis, recurrence, tumor microenvironment, epithelial-mesenchymal transition, and open labelling.
References
[1] Liu C, Zhang M, Yan X, Ni Y, Gong Y, Wang C, et al. (2023) Single-cell dissection of cellular and molecular features underlying human cervical squamous cell carcinoma initiation and progression. Sci Adv. 9(4):eadd8977. https://doi.org/10.1126/sciadv.add8977
[2] Zhang Y, Chen C, Liu Z, Guo H, Lu W, Hu W, et al. (2022) PABPC1-induced stabilization of IFI27 mRNA promotes angiogenesis and malignant progression in esophageal squamous cell carcinoma through exosomal miRNA-21-5p. J Exp Clin Cancer Res. 41(1):111. https://doi.org/10.1186/s13046-022-02339-9
[3] Chu T, Wang Z, Pe'er D, Danko CG. (2022). Cell type and gene expression deconvolution with BayesPrism enables Bayesian integrative analysis across bulk and single-cell RNA sequencing in oncology. Nat Cancer. 3(4):505-517. https://doi.org/10.1038/s43018-022-00356-3
[4] Sekiguchi S, Yorozu A, Okazaki F, Niinuma T, Takasawa A, Yamamoto E, et al. (2023) ACLP Activates Cancer-Associated Fibroblasts and Inhibits CD8+ T-Cell Infiltration in Oral Squamous Cell Carcinoma. Cancers (Basel). 15(17):4303. https://doi.org/10.3390/cancers15174303
[5] Wei WF, Zhou HL, Chen PY, Huang XL, Huang L, Liang LJ, et al. (2023) Cancer-associated fibroblast-derived PAI-1 promotes lymphatic metastasis via the induction of EndoMT in lymphatic endothelial cells. J Exp Clin Cancer Res. 42(1):160. https://doi.org/10.1186/s13046-023-02714-0
[6] Dumitru CS, Ceausu AR, Gaje NP, Suciu CS, Raica M. (2022). Proliferating Lymphatic Endothelial Cells as a Prognostic Marker in Head and Neck Squamous Cell Carcinoma. Int J Mol Sci, 23(17):9793. https://doi.org/10.3390/ijms23179793
[7] Dai J, Xi X, Liu Z, Wu W, Zhu S, Zhang X, et al. (2023) Single-cell sequencing of multi-region resolves geospatial architecture and therapeutic target of endothelial cells in esophageal squamous cell carcinoma. Clin Transl Med. 13(11):e1487. https://doi.org/10.1002/ctm2.1487
[8] Long SY, Shang L, Shi H, Zhao S, Cao J, He Y. (2023). The Future Landscape of Endothelial Cells Research in Psoriasis: Bibliometric Analysis and Literature Review. Clin Cosmet Investig Dermatol. 16(1):3107-3120. https://doi.org/10.2147/CCID.S435085
[9] Hanahan D, Weinberg RA. (2011). Hallmarks of cancer: the next generation. Cell. 144(5):646-74. https://doi.org/10.1016/j.cell.2011.02.013
[10] Li X, Wang C, Zhang H, Li Y, Hou D, Liu D, et al. (2023) circFNDC3B Accelerates Vasculature Formation and Metastasis in Oral Squamous Cell Carcinoma. Cancer Res. 83(9):1459-1475. https://doi.org/10.1158/0008-5472.CAN-22-2585
[11] Hakuno SK, Janson SGT, Trietsch MD, de Graaf M, de Jonge-Muller E, Crobach S, et al. (2023) Endoglin and squamous cell carcinomas. Front Med (Lausanne). 10(1):1112573. https://doi.org/10.3389/fmed.2023.1112573
[12] Su NW, Dai SH, Hsu K, Chang KM, Ko CC, Kao CW, et al. (2024) PD-L1-positive circulating endothelial progenitor cells associated with immune response to PD-1 blockade in patients with head and neck squamous cell carcinoma. Cancer Immunol Immunother. 73(1):3. https://doi.org/10.1007/s00262-023-03595-0
[13] Noh JJ, Kim MS, Cho YJ, Jeong SY, Lee YY, Ryu JY, et al. (2020) Anti-Cancer Activity of As4O6 and its Efficacy in a Series of Patient-Derived Xenografts for Human Cervical Cancer. Pharmaceutics. 12(10):987. https://doi.org/10.3390/pharmaceutics12100987
[14] Shimomura H, Sasahira T, Nakashima C, Kurihara-Shimomura M, Kirita T. (2019). Non-SMC Condensin I Complex Subunit H (NCAPH) Is Associated with Lymphangiogenesis and Drug Resistance in Oral Squamous Cell Carcinoma. J Clin Med. 9(1):72. https://doi.org/10.3390/jcm9010072
[15] Li C, Guan R, Li W, Wei D, Cao S, Xu C, et al. (2023) Single-cell RNA sequencing reveals tumor immune microenvironment in human hypopharygeal squamous cell carcinoma and lymphatic metastasis. Front Immunol. 14(1):1168191. https://doi.org/10.3389/fimmu.2023.1168191
[16] Zhang J, Lu T, Lu S, Ma S, Han D, Zhang K, et al. (2022) Single-cell analysis of multiple cancer types reveals differences in endothelial cells between tumors and normal tissues. Comput Struct Biotechnol J. 21(1):665-676. https://doi.org/10.1016/j.csbj.2022.12.049
[17] Chen P, Wang Y, Li J, Bo X, Wang J, Nan L, et al. (2021) Diversity and intratumoral heterogeneity in human gallbladder cancer progression revealed by single-cell RNA sequencing. Clin Transl Med. 11(6):e462. https://doi.org/10.1002/ctm2.462
[18] Tokozlu B, Yücel ÖÖ, Gültekin SE, Bozdağ LA. (2024). Epithelial mesenchymal transition and cancer stem cell markers in oral epithelial dysplasia and oral squamous cell carcinoma. Pol J Pathol. 75(4):305-314. https://doi.org/10.5114/pjp.2024.145818.
[19] Franz L, Nicolè L, Frigo AC, Ottaviano G, Gaudioso P, Saccardo T, et al. (2021) Epithelial-to-Mesenchymal Transition and Neoangiogenesis in Laryngeal Squamous Cell Carcinoma. Cancers (Basel). 13(13):3339. https://doi.org/10.3390/cancers13133339
[20] Xiao J, Song Y, Gao R, You M, Deng C, Tan G, et al. (2023) Changes of immune microenvironment in head and neck squamous cell carcinoma in 3D-4-culture compared to 2D-4-culture. J Transl Med. 21(1):771. https://doi.org/10.1186/s12967-023-04650-1
[21] He Z, Tian W, Wei Q, Xu J. (2022). Involvement of Fusobacterium nucleatum in malignancies except for colorectal cancer: A literature review. Front Immunol. 13(1):968649. https://doi.org/10.3389/fimmu.2022.968649
[22] Chen P, Wang Y, Li J, Bo X, Wang J, Nan L, et al. (2021) Diversity and intratumoral heterogeneity in human gallbladder cancer progression revealed by single-cell RNA sequencing. Clin Transl Med. 11(6):e462. https://doi.org/10.1002/ctm2.462
[23] Sun Q, Zhang T, Xiao Q, Mei B, Zhang X. (2022) Procyanidin B2 inhibits angiogenesis and cell growth in oral squamous cell carcinoma cells through the vascular endothelial growth factor (VEGF)/VEGF receptor 2 (VEGFR2) pathway. Bioengineered. 13(3): 6500-6508. https://doi.org/10.1080/21655979.2022.2033013
[24] Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr, et al. (2019) KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet, 394(10212): 1915-1928. https://doi.org/10.1016/S0140-6736(19)32591-7
[25] Ozer M, Sahin I . (2022). Nivolumab in Esophageal Squamous-Cell Carcinoma. N Engl J Med, 386(20):1958-1959. https://doi.org/10.1056/NEJMc2202880
Type
Published
Data Availability Statement
The data used in this study were obtained from the web of science (https://www.webofscience.com/wos), and the data and these results can also be obtained from the corresponding author, Junyi Zhou.
Issue
Section
License
Copyright (c) 2024 Life Conflux

This work is licensed under a Creative Commons Attribution 4.0 International License.







