A Mendelian randomization study investigating the causal relationship between 35 blood and urine metabolite biomarkers and postmenopausal osteoporosis
DOI:
https://doi.org/10.71321/ktnt2936Keywords:
Postmenopausal osteoporosis, Blood and urine biomarkers, Mendelian Randomization, Causal association, MetaboliteAbstract
Objective: This study intends to investigate the causal association between 35 blood and urine biomarkers and postmenopausal osteoporosis (PMOP) through two-way Mendelian randomization analysis.
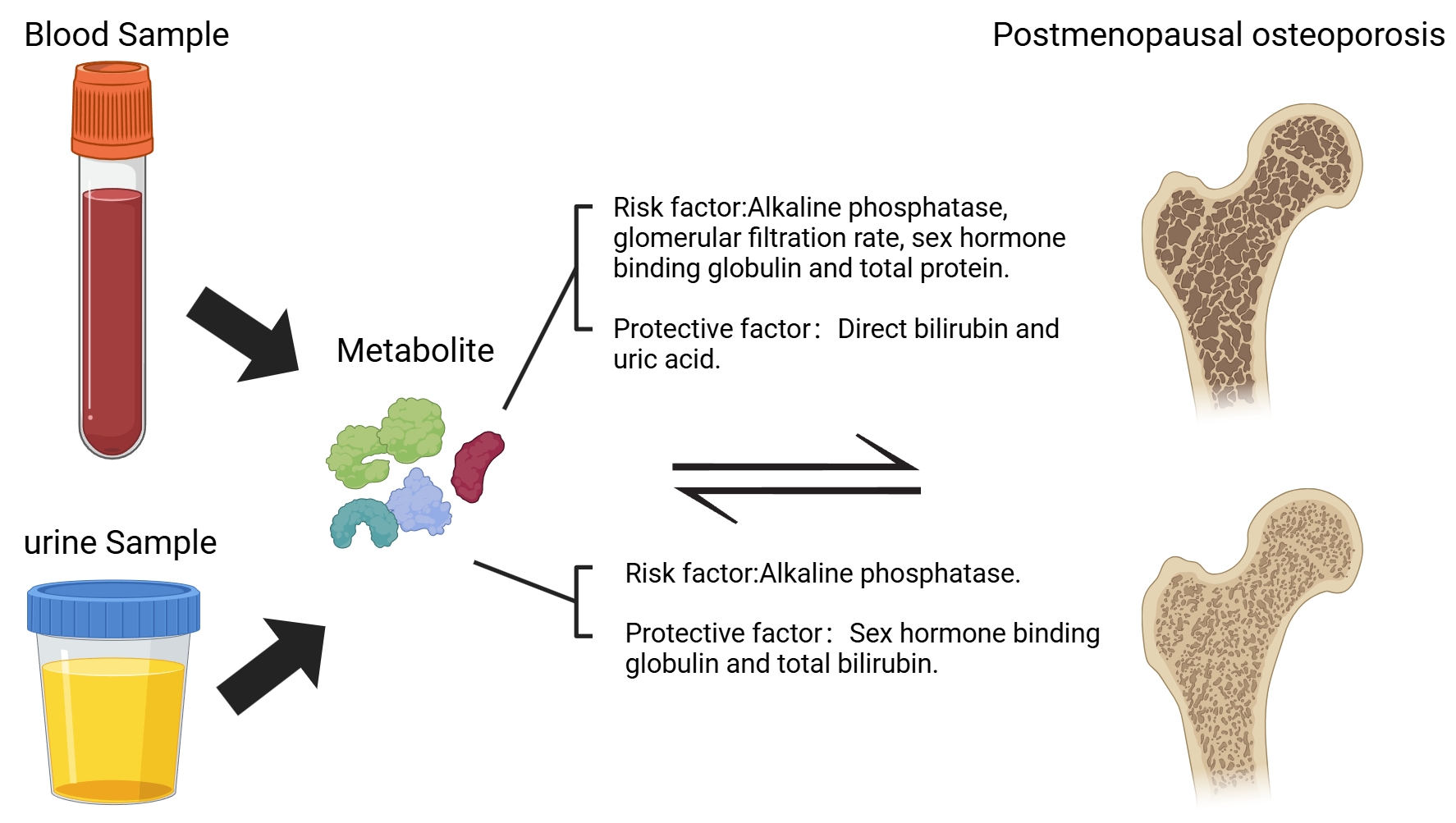
Methods: This study adopted a two-way Mendelian randomization analysis, with data sourced from the UK Biobank and the Finnish Biobank Study. Among them, the R12 dataset of the Finnish Biobank Study was used as the test set, and the R11 dataset as the validation set. The study regarded 35 biomarkers as exposure factors and PMOP (a condition characterized by decreased bone density after menopause) as the outcome variable. It was analyzed through methods such as the inverse variance weighting method, the weighted median method, and MR-Egger regression, and combined with the MR-PRESSO test to exclude the influence of pleiotropy.
Results: In the positive direction analysis, alkaline phosphatase, glomerular filtration rate, sex hormone-binding globulin, and total protein showed statistical significance in both the test set and the validation set, and they were all risk factors for PMOP. Direct bilirubin and uric acid demonstrated statistical significance in both the test and validation sets, and they served as protective factors against PMOP. In the negative direction analysis, alkaline phosphatase showed statistical significance in both the test set and the validation set, being a positive result for PMOP; sex hormone-binding globulin and total bilirubin showed statistical significance in both the test set and the validation set, being negative results for PMOP.
Conclusion: Employing bidirectional Mendelian randomization methodology, this investigation elucidated the causal relationships between multiple hematological and urinary biomarkers and PMOP. The results provide promising biomarker candidates for future diagnostic and therapeutic strategies targeting PMOP, while simultaneously establishing a robust framework for subsequent exploration of its underlying pathophysiological mechanisms.
References
[1] Arceo-Mendoza RM, & Camacho PM. (2021). Postmenopausal Osteoporosis: Latest Guidelines. Endocrinol Metab Clin North Am, 50(2), 167-178. https://doi.org/10.1016/j.ecl.2021.03.009
[2] Walker MD, & Shane E. (2023). Postmenopausal Osteoporosis. N Engl J Med, 389(21), 1979-1991. https://doi.org/10.1056/NEJMcp2307353
[3] Zhang L, Zheng YL, Wang R, Wang XQ, & Zhang H. (2022). Exercise for osteoporosis: A literature review of pathology and mechanism. Front Immunol, 13, 1005665. https://doi.org/10.3389/fimmu.2022.1005665
[4] Migliorini F, Maffulli N, Spiezia F, Peretti GM, Tingart M, & Giorgino R. (2021). Potential of biomarkers during pharmacological therapy setting for postmenopausal osteoporosis: a systematic review. J Orthop Surg Res, 16(1), 351. https://doi.org/10.1186/s13018-021-02497-0
[5] Marriott RJ, Murray K, Adams RJ, Antonio L, Ballantyne CM, Bauer DC, et al. (2023). Factors Associated With Circulating Sex Hormones in Men : Individual Participant Data Meta-analyses. Ann Intern Med, 176(9), 1221-1234. https://doi.org/10.7326/m23-0342
[6] Sadhukhan S, Sethi S, Rajender S, Mithal A, & Chattopadhyay N. (2023). Understanding the characteristics of idiopathic osteoporosis by a systematic review and meta-analysis. Endocrine, 82(3), 513-526. https://doi.org/10.1007/s12020-023-03505-5
[7] Sekula P, Del Greco MF, Pattaro C, & Köttgen A. (2016). Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J Am Soc Nephrol, 27(11), 3253-3265. https://doi.org/10.1681/asn.2016010098
[8] Li J, Wang W, Liu F, Qiu L, Ren Y, Li M, et al. (2024). Genetically predicted 1091 blood metabolites and 309 metabolite ratios in relation to risk of type 2 diabetes: a Mendelian randomization study. Front Genet, 15, 1356696. https://doi.org/10.3389/fgene.2024.1356696
[9] Xu M, Zheng J, Hou T, Lin H, Wang T, Wang S, et al. (2022). SGLT2 Inhibition, Choline Metabolites, and Cardiometabolic Diseases: A Mediation Mendelian Randomization Study. Diabetes Care, 45(11), 2718-2728. https://doi.org/10.2337/dc22-0323
[10] Pan T, Bai L, Zhu D, Wei Y, Zhao Q, Feng F, et al. (2024). The causal relationship between genetically predicted blood metabolites and idiopathic pulmonary fibrosis: A bidirectional two-sample Mendelian randomization study. PLoS One, 19(4), e0300423. https://doi.org/10.1371/journal.pone.0300423
[11] Sinnott-Armstrong N, Tanigawa Y, Amar D, Mars N, Benner C, Aguirre M, et al. (2021). Genetics of 35 blood and urine biomarkers in the UK Biobank. Nat Genet, 53(2), 185-194. https://doi.org/10.1038/s41588-020-00757-z
[12] Fan Z, Zhao J, Chen J, Hu W, Ma J, & Ma X. (2024). Causal associations of osteoporosis with stroke: a bidirectional Mendelian randomization study. Osteoporos Int, 35(12), 2127-2135. https://doi.org/10.1007/s00198-024-07235-w
[13] Chen D, Xu W, Wen Y, Tan X, & Liu J. (2024). Causal relationship analysis between 35 blood/urine metabolites and gastroesophageal reflux disease: A Mendelian randomization combined meta-analysis study. Medicine (Baltimore), 103(32), e39248. https://doi.org/10.1097/md.0000000000039248
[14] An W, Zhao C, Wang Y, Zhang Y, & Qiao Z. (2024). Identifying causal relationships between 35 blood and urine biomarkers and urologic cancers: MR-meta combined with Bayesian colocalization Mendelian randomization analysis. Discov Oncol, 15(1), 617. https://doi.org/10.1007/s12672-024-01493-0
[15] Birney E. (2022). Mendelian Randomization. Cold Spring Harb Perspect Med, 12(4). https://doi.org/10.1101/cshperspect.a041302
[16] Pierce BL, Ahsan H, & Vanderweele TJ. (2011). Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol, 40(3), 740-752. https://doi.org/10.1093/ije/dyq151
[17] Burgess S, & Thompson SG. (2017). Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol, 32(5), 377-389. https://doi.org/10.1007/s10654-017-0255-x
[18] Vitek L, Hinds TD, Jr., Stec DE, & Tiribelli C. (2023). The physiology of bilirubin: health and disease equilibrium. Trends Mol Med, 29(4), 315-328. https://doi.org/10.1016/j.molmed.2023.01.007
[19] Kimball JS, Johnson JP, & Carlson DA. (2021). Oxidative Stress and Osteoporosis. J Bone Joint Surg Am, 103(15), 1451-1461. https://doi.org/10.2106/jbjs.20.00989
[20] Xu R, Lian D, Xie Y, Mu L, Wu Y, Chen Z, et al. (2023). Relationship between serum uric acid levels and osteoporosis. Endocr Connect, 12(11). https://doi.org/10.1530/ec-23-0040
[21] Yang K, Li J, & Tao L. (2022). Purine metabolism in the development of osteoporosis. Biomed Pharmacother, 155, 113784. https://doi.org/10.1016/j.biopha.2022.113784
[22] Feng XJ, Zhou WJ, Zhang J, Zhang YD, Yu XN, & Yu F. (2024). [Research progress of novel bone turnover markers in osteoporosis]. Zhonghua Yu Fang Yi Xue Za Zhi, 58(12), 2045-2055. https://doi.org/10.3760/cma.j.cn112150-20240710-00556
[23] Zhao S, Gu J, Tian Y, Wang R, & Li W. (2024). Low levels of sex hormone-binding globulin predict an increased breast cancer risk and its underlying molecular mechanisms. Open Life Sci, 19(1), 20220822. https://doi.org/10.1515/biol-2022-0822
Type
Published
Data Availability Statement
The data supporting this study are included in the manuscript or its supplementary materials. Publicly accessible datasets were utilized for this analysis and are available at the following repositories: (https://www.finngen.fi/en) and (https://www.ukbiobank.ac.uk/).
Issue
Section
License
Copyright (c) 2024 Life Conflux

This work is licensed under a Creative Commons Attribution 4.0 International License.







