Impact of heavy metals on allostatic load in adults: a NHANES study
DOI:
https://doi.org/10.71321/3b9xjm29Keywords:
Urinary metals, Allostatic load score, National health and nutrition examination survey, Machine LearningAbstract
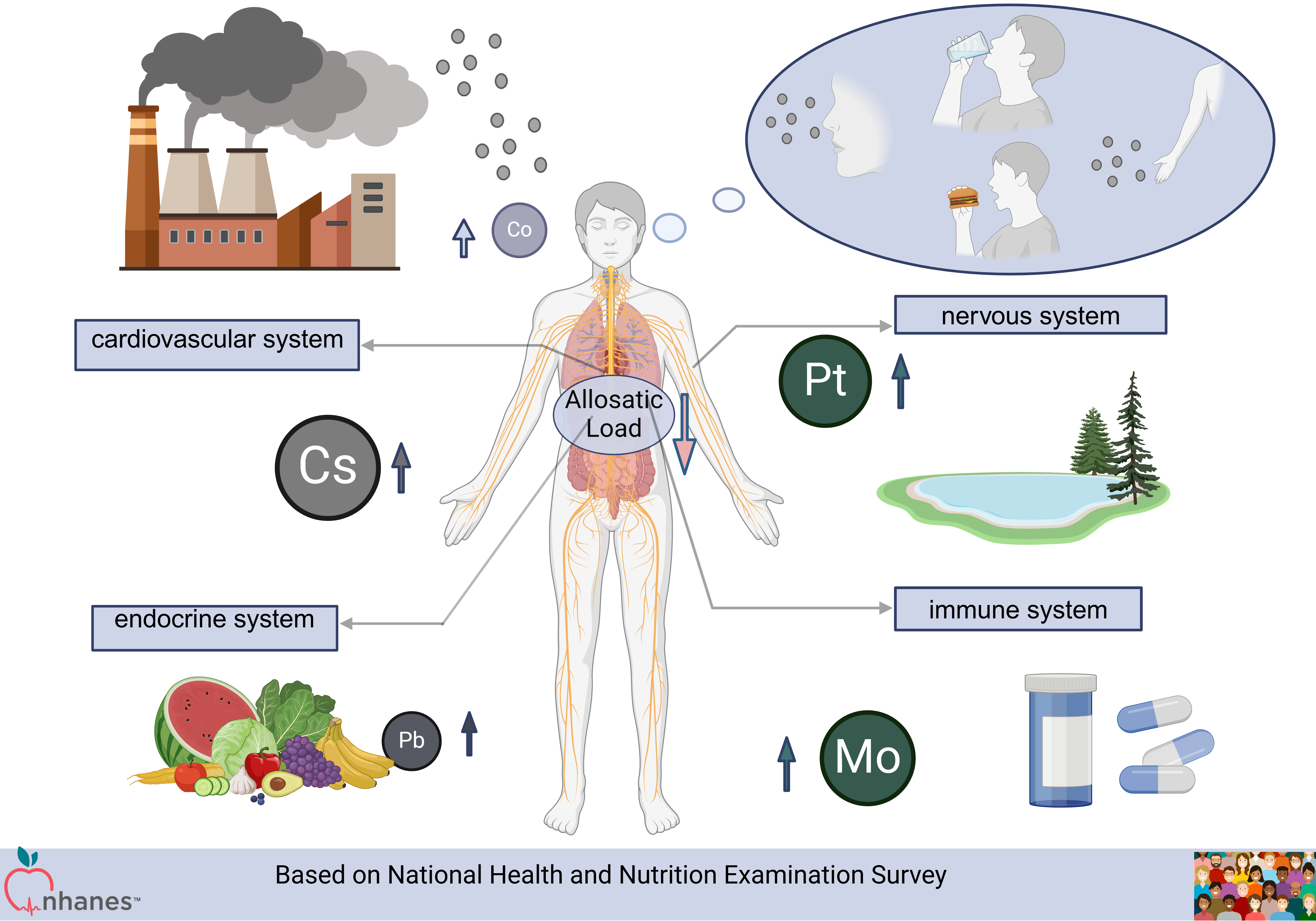
Background: Allostatic load, defined as the cumulative strain resulting from the chronic stress response, is associated with adverse health outcomes. Heavy metals, prevalent in various environmental pollutants, exert cumulative effects on the human body through exposure via water or food sources. However, the relationship between heavy metals and allostatic load remains poorly understood. The aim of this study is to examine the association between urinary metal concentrations and allostatic load.
Methods: This study analyzed data from 4,231 adult participants in the National Health and Nutrition Examination Survey (NHANES) conducted between 2005 and 2010. We employed linear regression analysis, Bayesian kernel machine regression (BKMR), weighted quantile sum (WQS), and quantile g-computation (qgcomp) to investigate the associations between twelve urinary metals and allostatic load. Additionally, we developed K-nearest neighbors (KNN), random forest (RF), and XGBoost models to predict allostatic load scores (ALS).
Results: Linear regression analysis indicated that the combined effects of the twelve urinary metals were negatively correlated with allostatic load. WQS, qgcomp, and BKMR analyses identified cesium (Cs), molybdenum (Mo), lead (Pb), platinum (Pt), and cobalt (Co) as the primary influencing factors (all p-values < 0.05). Furthermore, when predicting ALS based on heavy metal exposure, the random forest model outperformed the other machine learning algorithms, with a root mean square error (RMSE) of 2.377428, compared to 2.501523 for KNN and 2.377733 for XGBoost.
Conclusion: Our findings indicate that urinary metal concentrations are negatively associated with allostatic load, with Cs, Mo, Pt, Pb, and Co showing the most significant negative correlations. Further research is necessary to explore the causal relationships and underlying mechanisms. Additionally, our analysis demonstrated that the random forest model was the most effective for ALS prediction.
References
[1] Sterling, P., & Eyer, J. (1988). Allostasis: A New Paradigm to Explain Arousal Pathology. handbook of life stress cognition & health.
[2] Barrett, M., Wilcox, N. S., Huang, A., Levy, R., Demissei, B., Narayan, V., et al. (2022). Bearing allostatic load: insights into a more equitable future within cardio-oncology. Trends Mol Med, 28(12), 1040-1049. https://doi.org/10.1016/j.molmed.2022.09.006
[3] Parker, H. W., Abreu, A. M., Sullivan, M. C., & Vadiveloo, M. K. (2022). Allostatic Load and Mortality: A Systematic Review and Meta-Analysis. American journal of preventive medicine, 63(1), 131-140.
[4] Renu, K., Chakraborty, R., Haritha, M., Rajeshwari, K., & Abilash, V. G. (2021). Molecular mechanism of heavy metals (Lead, Chromium, Arsenic, Mercury, Nickel and Cadmium) induced hepatotoxicity – A review. Chemosphere, 129735.
[5] Mcewen, B. S. (2010). Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840(NEUROIMMUNOMODULATION: MOLECULAR ASPECTS), 33-44.
[6] Borrell, L. N., Dallo, F. J., & Nguyen, N. (2010). Racial/ethnic disparities in all-cause mortality in U.S. adults: the effect of allostatic load. Public Health Rep, 125(6), 810-816. https://doi.org/10.1177/003335491012500608
[7] Duong, M. T., Bingham, B. A., Aldana, P. C., Chung, S. T., & Sumner, A. E. (2016). Variation in the Calculation of Allostatic Load Score: 21 Examples from NHANES. Journal of Racial & Ethnic Health Disparities, 4(3), 1-7.
[8] Beckie, & T., M. (2012). A systematic review of allostatic load, health, and health disparities. Biological Research for Nursing, 14(4), 311-346.
[9] Juster, R. P., McEwen, B. S., & Lupien, S. J. (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev, 35(1), 2-16. https://doi.org/10.1016/j.neubiorev.2009.10.002
[10] Huang, Q., Wan, J., Nan, W., Li, S., He, B., & Peng, Z. (2024). Association between manganese exposure in heavy metals mixtures and the prevalence of sarcopenia in US adults from NHANES 2011–2018. Journal of Hazardous Materials, (Feb.15), 464.
[11] Guidi, J., Lucente, M., Sonino, N., & Fava, G. A. (2021). Allostatic Load and Its Impact on Health: A Systematic Review. Psychother Psychosom, 90(1), 11-27. https://doi.org/10.1159/000510696
[12] Mieiro, C. L., Duarte, A. C., Pereira, M. E., & Pacheco, M. (2011). Mercury accumulation patterns and biochemical endpoints in wild fish (Liza aurata): a multi-organ approach. Ecotoxicol Environ Saf, 74(8), 2225-2232. https://doi.org/10.1016/j.ecoenv.2011.08.011
[13] Lv, Y. J., Wei, Q. Z., Zhang, Y. C., Huang, R., Li, B. S., Tan, J. B., et al. (2019). Low-dose cadmium exposure acts on rat mesenchymal stem cells via RANKL/OPG and downregulate osteogenic differentiation genes. Environ Pollut, 249, 620-628. https://doi.org/10.1016/j.envpol.2019.03.027
[14] Javed, M., Ahmad, M. I., Usmani, N., & Ahmad, M. (2017). Multiple biomarker responses (serum biochemistry, oxidative stress, genotoxicity and histopathology) in Channa punctatus exposed to heavy metal loaded waste water. Sci Rep, 7(1), 1675. https://doi.org/10.1038/s41598-017-01749-6
[15] Slade, G. D., Sanders, A. E., & By, K. (2012). Role of Allostatic Load in Sociodemographic Patterns of Pain Prevalence in the U.S. Population. Journal of Pain Official Journal of the American Pain Society, 13(7).
[16] Frei, R., Haile, S. R., Mutsch, M., & Rohrmann, S. (2015). Relationship of Serum Vitamin D Concentrations and Allostatic Load as a Measure of Cumulative Biological Risk among the US Population: A Cross-Sectional Study. PLoS One, 10(10), e0139217. https://doi.org/10.1371/journal.pone.0139217
[17] Wheeler, D. C., Rustom, S., Carli, M., Whitehead, T. P., & Metayer, C. (2021). Assessment of Grouped Weighted Quantile Sum Regression for Modeling Chemical Mixtures and Cancer Risk. International Journal of Environmental Research and Public Health, 18(2), 504.
[18] Keil, A. P., Buckley, J. P., O'Brien, K. M., Ferguson, K. K., Zhao, S., & White, A. J. (2020). A Quantile-Based G-Computation Approach to Addressing the Effects of Exposure Mixtures. Environmental Health Perspectives, 128.
[19] Bobb, J. F., Valeri, L., Henn, B. C., Christiani, D. C., & Coull, B. A. (2014). Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics, 16(3), 493.
[20] Zha, B., Liu, Y., & Xu, H. (2023). Associations of mixed urinary metals exposure with metabolic syndrome in the US adult population. Chemosphere, 344, 140330. https://doi.org/10.1016/j.chemosphere.2023.140330
[21] Breiman, L. (2001). Random Forests. Machine Learning, 45(1), 5-32. https://doi.org/10.1023/A:1010933404324
[22] Archer, K. J., & Kimes, R. V. (2008). Empirical characterization of random forest variable importance measures. Computational Statistics & Data Analysis, 52(4), 2249-2260. https://doi.org/10.1016/j.csda.2007.08.015
[23] Tan, K., Ma, W., Wu, F., & Du, Q. (2019). Random forest-based estimation of heavy metal concentration in agricultural soils with hyperspectral sensor data. Environ Monit Assess, 191(7), 446. https://doi.org/10.1007/s10661-019-7510-4
[24] Dai, P., Chang, W., Xin, Z., Cheng, H., Ouyang, W., & Luo, A. (2021). Retrospective Study on the Influencing Factors and Prediction of Hospitalization Expenses for Chronic Renal Failure in China Based on Random Forest and LASSO Regression. Front Public Health, 9, 678276. https://doi.org/10.3389/fpubh.2021.678276https://doi.org/10.3389/fpubh.2021.678276
[25] Ellis, K., Kerr, J., Godbole, S., Lanckriet, G., Wing, D., & Marshall, S. (2014). A random forest classifier for the prediction of energy expenditure and type of physical activity from wrist and hip accelerometers. Physiol Meas, 35(11), 2191-2203. https://doi.org/10.1088/0967-3334/35/11/2191
[26] Guo, J., Su, L., Zhao, X., Xu, Z., & Chen, G. (2016). Relationships between urinary antimony levels and both mortalities and prevalence of cancers and heart diseases in general US population, NHANES 1999–2010. Science of The Total Environment, 571, 452-460. https://doi.org/10.1016/j.scitotenv.2016.07.011
[27] Yang, A. M., Lo, K., Zheng, T. Z., Yang, J. L., & Liu, S. M. (2020). Environmental heavy metals and cardiovascular diseases: Status and future direction. Chronic Diseases and Translational Medicine.
[28] Nguyen, H. D., Oh, H., Hoang, N. H. M., & Kim, M. S. (2021). Association between heavy metals, high-sensitivity C-reaction protein and 10-year risk of cardiovascular diseases among adult Korean population. Nature Publishing Group, (1).
[29] Hoffmann, B., Moebus, S., Dragano, N., Stang, A., Mohlenkamp, S., Schmermund, A., et al. (2009). Chronic residential exposure to particulate matter air pollution and systemic inflammatory markers. Environ Health Perspect, 117(8), 1302-1308. https://doi.org/10.1289/ehp.0800362
[30] Manens, L., Grison, S., Bertho, J. M., Lestaevel, P., Gueguen, Y., Benderitter, M., et al. (2016). Chronic exposure of adult, postnatal and in utero rat models to low-dose 137Cesium: impact on circulating biomarkers. J Radiat Res, 57(6), 607-619. https://doi.org/10.1093/jrr/rrw067
[31] Li, A., Li, Y., Mei, Y., Zhao, J., Zhou, Q., Li, K., et al. (2023). Associations of metals and metals mixture with lipid profiles: A repeated-measures study of older adults in Beijing. Chemosphere, 319, 137833. https://doi.org/10.1016/j.chemosphere.2023.137833
[32] Xu, C., Weng, Z., Zhang, L., Xu, J., Dahal, M., Basnet, T. B., et al. (2021). HDL cholesterol: A potential mediator of the association between urinary cadmium concentration and cardiovascular disease risk. Ecotoxicol Environ Saf, 208, 111433. https://doi.org/10.1016/j.ecoenv.2020.111433
[33] Padilla, M. A., Elobeid, M., Ruden, D. M., & Allison, D. B. (2010). An examination of the association of selected toxic metals with total and central obesity indices: NHANES 99-02. Int J Environ Res Public Health, 7(9), 3332-3347. https://doi.org/10.3390/ijerph7093332
[34] Giedroc, D. P., & Arunkumar, A. I. (2007). Metal sensor proteins: nature's metalloregulated allosteric switches. Dalton Trans, 10.1039/b706769k(29), 3107-3120. https://doi.org/10.1039/b706769k
[35] Tat'ianenko, L. V., Gromova, L. A., & Moshkovskiĭ, I. (1984). [The effect of biologically active compounds on the enzymatic activity of sarcoplasmic reticulum (Ca2+,Mg2+)-dependent ATPase transformed by synthetic phospholipids]. Mol Biol (Mosk), 18(2), 504-511. (Vliianie biologicheski aktivnykh soedineniĭ na fermentativnuiu aktivnost' rekonstruirovannoĭ sinteticheskimi fosfolipidami (Ca2+,Mg2+)-zavisomoĭ ATP-azy sarkoplazmaticheskogo retikuluma.)
Type
Published
Data Availability Statement
The data that support the findings of this study are openly available in NHANES (NHANES Questionnaires, Datasets, and Related Documentation (cdc.gov)). Additional data is provided by reference to the Methods section or upon reasonable request.
Issue
Section
License
Copyright (c) 2025 Life Conflux

This work is licensed under a Creative Commons Attribution 4.0 International License.







