The role of IL-17 family in the process of pulmonary fibrosis
DOI:
https://doi.org/10.71321/tg620c27Keywords:
interleukin-17 (IL-17) family, IL-17 receptor, pulmonary fibrosisAbstract
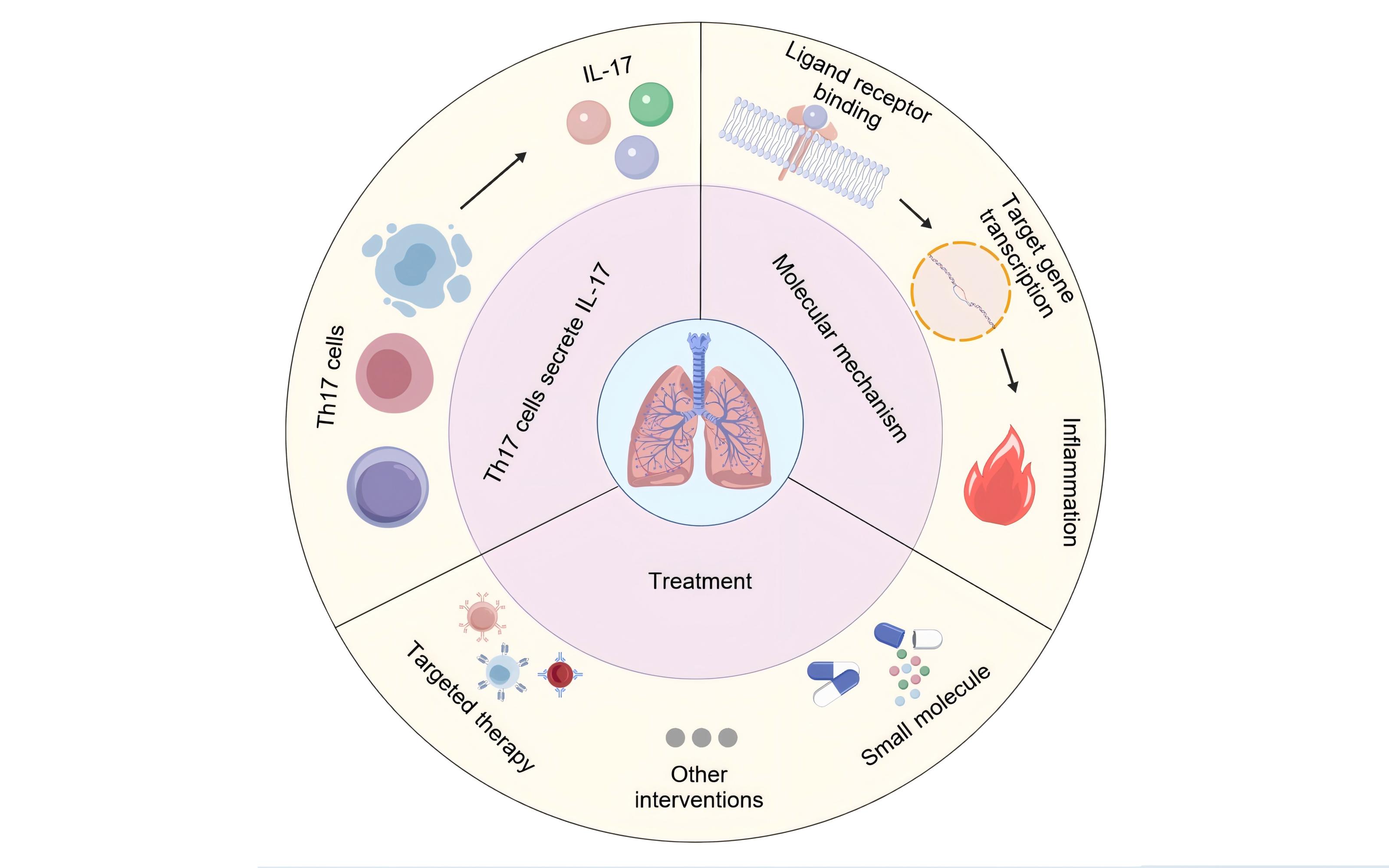
Pulmonary fibrosis is a serious lung disease characterized by the destruction of alveolar structures and excessive proliferation of fibrous tissue. The interleukin-17 (IL-17) family consists of six members (IL-17A-17F), which play a crucial role in the occurrence and development of pulmonary fibrosis. The IL-17 family drives pulmonary fibrosis through multiple mechanisms such as pro-inflammatory cytokines, immune cell recruitment, and fibroblast activation. IL-17A is the core molecule, while other members participate in the disease process through synergistic or independent pathways. Targeting the IL-17 signaling axis provides a new strategy for the treatment of pulmonary fibrosis. This article summarizes the effects of IL-17 on pulmonary inflammation response and fibrosis process through literature review, as well as its possible involvement in signal transduction molecular mechanisms, and explores its potential as a therapeutic target, providing theoretical basis for future research aimed at regulating IL-17 expression and function to treat related diseases.
References
[1]Bridges JP, Vladar EK, Kurche JS, Krivoi A, Stancil IT, Dobrinskikh E, et al. (2025). Progressive lung fibrosis: reprogramming a genetically vulnerable bronchoalveolar epithelium. Journal of Clinical Investigation, 135(1). https://doi.org/10.1172/JCI183836
[2]Chen T, Sun W, Xu Z-j. (2024). The immune mechanisms of acute exacerbations of idiopathic pulmonary fibrosis. Frontiers in Immunology, 15. https://doi.org/10.3389/fimmu.2024.1450688
[3]Mackintosh JA, Keir G, Troy LK, Holland AE, Grainge C, Chambers DC, et al. (2024). Treatment of idiopathic pulmonary fibrosis and progressive pulmonary fibrosis: A position statement from the Thoracic Society of Australia and New Zealand 2023 revision. Respirology, 29(2):105-35. https://doi.org/10.1111/resp.14656
[4]Han X, Su X, Che M, Liu L, Nie P, Wang S. (2025). Identification and Expression Analyses of IL-17/IL-17R Gene Family in Snakehead (Channa argus) Following Nocardia seriolae Infection. Genes, 16(3). https://doi.org/10.3390/genes16030253
[5]Huangfu L, Li R, Huang Y, Wang S. (2023). The IL-17 family in diseases: from bench to bedside. Signal Transduction and Targeted Therapy, 8(1). https://doi.org/10.1038/s41392-023-01620-3
[6]Chen S, Fan H, Ran C, Hong Y, Feng H, Yue Z, et al. (2024). The IL-17 pathway intertwines with neurotrophin and TLR/IL-1R pathways since its domain shuffling origin. Proceedings of the National Academy of Sciences of the United States of America, 121(19). https://doi.org/10.1073/pnas.2400903121
[7]Kolls JK, Lindén A. (2004). Interleukin-17 Family Members and Inflammation. Immunity, 21(4):467-76. https://doi.org/10.1016/j.immuni.2004.08.018
[8]Nie Y-J, Wu S-H, Xuan Y-H, Yan G. (2022). Role of IL-17 family cytokines in the progression of IPF from inflammation to fibrosis. Military Medical Research, 9(1). https://doi.org/10.1186/s40779-022-00382-3
[9]Tollenaere MAX, Hebsgaard J, Ewald DA, Lovato P, Garcet S, Li X, et al. (2021). Signalling of multiple interleukin (IL)‐17 family cytokines via IL‐17 receptor A drives psoriasis‐related inflammatory pathways. British Journal of Dermatology, 185(3):585-94. https://doi.org/10.1111/bjd.20090
[10]Wen Y, Chen Q, Wang H, Xie S, Chen H, Yao W, et al. (2024). Contribution of IL-17C-mediated macrophage polarization to Type 17 inflammation in neutrophilic asthma. Cell Communication and Signaling, 22(1). https://doi.org/10.1186/s12964-024-01937-8
[11]Mu X, Gu R, Tang M, Wu X, He W, Nie X. (2024). IL-17 in wound repair: bridging acute and chronic responses. Cell Communication and Signaling, 22(1). https://doi.org/10.1186/s12964-024-01668-w
[12]Knizkova D, Pribikova M, Draberova H, Semberova T, Trivic T, Synackova A, et al. (2022). CMTM4 is a subunit of the IL-17 receptor and mediates autoimmune pathology. Nature Immunology, 23(11):1644-52. https://doi.org/10.1038/s41590-022-01325-9
[13]Herjan T, Hong L, Bubenik J, Bulek K, Qian W, Liu C, et al. (2018). IL-17-receptor-associated adaptor Act1 directly stabilizes mRNAs to mediate IL-17 inflammatory signaling. Nature Immunology, 19(4):354-65. https://doi.org/10.1038/s41590-018-0071-9
[14]Brackman LC, Jung MS, Green EH, Joshi N, Revetta FL, McClain MS, et al. (2024)IL-17 signaling protects against Helicobacter pylori- induced gastric cancer. Gut Microbes, 16(1).https://doi.org/10.1080/19490976.2024.2430421
[15]Enzel D, Kriventsov M, Sataieva T, Malygina V. (2024). Cellular and Molecular Genetic Mechanisms of Lung Fibrosis Development and the Role of Vitamin D: A Review. International Journal of Molecular Sciences, 25(16). https://doi.org/10.3390/ijms25168946
[16]Meehan EV, Wang K. (2022). Interleukin-17 Family Cytokines in Metabolic Disorders and Cancer. Genes, 13(9). https://doi.org/10.3390/genes13091643
[17]Gouda MM, Bhandary YP. (2019). Acute Lung Injury: IL-17A-Mediated Inflammatory Pathway and Its Regulation by Curcumin. Inflammation, 42(4), 1160–1169. https://doi.org/10.1007/s10753-019-01010-4
[18]Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, et al. (2010). Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. Journal of experimental medicine, 207(3), 535–552. https://doi.org/10.1084/jem.20092121
[19]Liu C, Zhu L, Fukuda K, Ouyang S, Chen X, Wang C, et al. (2017). The flavonoid cyanidin blocks binding of the cytokine interleukin-17A to the IL-17RA subunit to alleviate inflammation in vivo. Science Signaling, 10(467). https://doi.org/10.1126/scisignal.aaf8823
[20]Golebski K, Ros XR, Nagasawa M, van Tol S, Heesters BA, Aglmous H, et al. (2019). IL-1β, IL-23, and TGF-β drive plasticity of human ILC2s towards IL-17-producing ILCs in nasal inflammation. Nature Communications, 10(1). https://doi.org/10.1038/s41467-019-09883-7
[21]Glatt S, Baeten D, Baker T, Griffiths M, Ionescu L, Lawson ADG, et al. (2018). Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Annals of the Rheumatic Diseases, 77(4):523-32. https://doi.org/10.1136/annrheumdis-2017-212127
[22]Chang Seon H, Reynolds Joseph M, Pappu Bhanu P, Chen G, Martinez Gustavo J, Dong C. (2011). Interleukin-17C Promotes Th17 Cell Responses and Autoimmune Disease via Interleukin-17 Receptor E. Immunity, 35(4):611-21. https://doi.org/10.1016/j.immuni.2011.09.010
[23]Reynolds Joseph M, Lee Y-H, Shi Y, Wang X, Angkasekwinai P, Nallaparaju Kalyan C, et al. (2015). Interleukin-17B Antagonizes Interleukin-25-Mediated Mucosal Inflammation. Immunity, 42(4):692-703. https://doi.org/10.1016/j.immuni.2015.03.008
[24]Shabgah AG, Fattahi E, Shahneh FZ. (2014). Interleukin-17 in human inflammatory diseases. Advances in Dermatology and Allergology, 31 (4):256-61. https://doi.org/10.5114/pdia.2014.40954
[25]Khader S, Gasse P, Riteau N, Vacher R, Michel M-L, Fautrel A, et al. (2011). IL-1 and IL-23 Mediate Early IL-17A Production in Pulmonary Inflammation Leading to Late Fibrosis. PLoS ONE, 6(8). https://doi.org/10.1371/journal.pone.0023185
[26]He Q, Cao J, Zhang M, Feng C. (2024). IL-17 in plasma and bronchoalveolar lavage fluid in non-neutropenic patients with invasive pulmonary aspergillosis. Frontiers in Cellular and Infection Microbiology, 14. https://doi.org/10.3389/fcimb.2024.1402888
[27]Jiang G, Liu CT, Zhang WD. (2018). IL‑17A and GDF15 are able to induce epithelial‑mesenchymal transition of lung epithelial cells in response to cigarette smoke. Experimental and Therapeutic Medicine, 16(1), 12-20. https://doi.org/10.3892/etm.2018.6145
[28]Sisto M, Lorusso L, Tamma R, Ingravallo G, Ribatti D, Lisi S. (2019). Interleukin-17 and -22 synergy linking inflammation and EMT-dependent fibrosis in Sjögren’s syndrome. Clinical and Experimental Immunology. 2019;198(2):261-72. https://doi.org/10.1111/cei.13337
[29]Qu Z, Dou W, Zhang K, Duan L, Zhou D, Yin S. (2022). IL-22 inhibits bleomycin-induced pulmonary fibrosis in association with inhibition of IL-17A in mice. Arthritis Research & Therapy, 24(1). https://doi.org/10.1186/s13075-022-02977-6
[30]Sisto M, Lisi S. (2023). Targeting Interleukin-17 as a Novel Treatment Option for Fibrotic Diseases. Journal of Clinical Medicine, 13(1). https://doi.org/10.3390/jcm13010164
[31]Liang M, Wang J, Chu H, Zhu X, He H, Liu Q, et al. (2013). Interleukin-22 Inhibits Bleomycin-Induced Pulmonary Fibrosis. Mediators of Inflammation,2013:1-11. https://doi.org/10.1155/2013/209179
[32]Gurczynski SJ, Moore BB. (2018). IL-17 in the lung: the good, the bad, and the ugly. American journal of physiology. Lung cellular and molecular physiology, 314(1), L6–L16. https://doi.org/10.1152/ajplung.00344.2017
[33]Dong Z, Yang Y, Zhang T, Li Y, Kang Q, Lei W,et al. (2013). siRNA-Act1 inhibits the function of IL-17 on lung fibroblasts via the NF-κB pathway. Respiration; international review of thoracic diseases, 86(4), 332–340. https://doi.org/10.1159/000348403
[34]Sønder SU, Saret S, Tang W, Sturdevant DE, Porcella SF, Siebenlist U. (2011). IL-17-induced NF-κB Activation via CIKS/Act1. Journal of Biological Chemistry, 286(15):12881-90. https://doi.org/10.1074/jbc.M110.199547
[35]Huang L. (2024). The role of IL-17 family cytokines in cardiac fibrosis. Frontiers in cardiovascular medicine, 11. https://doi.org/10.3389/fcvm.2024.1470362
[36]Morrow KN, Coopersmith CM, Ford ML. (2019). IL-17, IL-27, and IL-33: A Novel Axis Linked to Immunological Dysfunction During Sepsis. Frontiers in Immunology, 10. https://doi.org/10.3389/fimmu.2019.01982
[37]Krohn S, Nies JF, Kapffer S, Schmidt T, Riedel JH, Kaffke A, et al. (2018). IL-17C/IL-17 Receptor E Signaling in CD4+ T Cells Promotes TH17 Cell-Driven Glomerular Inflammation. Journal of the American Society of Nephrology : JASN, 29(4), 1210–1222. https://doi.org/10.1681/ASN.2017090949
[38]Vandeghinste N, Klattig J, Jagerschmidt C, Lavazais S, Marsais F, Haas JD, et al. (2018). Neutralization of IL-17C Reduces Skin Inflammation in Mouse Models of Psoriasis and Atopic Dermatitis. Journal of Investigative Dermatology, 138(7):1555-63. https://doi.org/10.1016/j.jid.2018.01.036
[39]Naglik JR, Conti HR, Whibley N, Coleman BM, Garg AV, Jaycox JR, et al. (2015). Signaling through IL-17C/IL-17RE Is Dispensable for Immunity to Systemic, Oral and Cutaneous Candidiasis. Plos One, 10(4). https://doi.org/10.1371/journal.pone.0122807
[40]Chen S, Zhang X, Yang C, Wang S, Shen H. (2022). Essential role of IL-17 in acute exacerbation of pulmonary fibrosis induced by non-typeable Haemophilus influenzae. Theranostics, 12(11):5125-37. https://doi.org/10.7150/thno.74809
[41]Yildirim AÖ, Vella G, Ritzmann F, Wolf L, Kamyschnikov A, Stodden H, et al. (2021). IL-17C contributes to NTHi-induced inflammation and lung damage in experimental COPD and is present in sputum during acute exacerbations. Plos One, 16(1). https://doi.org/10.1371/journal.pone.0243484
[42]Liu X, Sun S, Liu D. (2020). IL-17D: A Less Studied Cytokine of IL-17 Family. International Archives of Allergy and Immunology, 181(8):618-23. https://doi.org/10.1159/000508255
[43]Xu X, Luo S, Li B, Dai H, Zhang J. (2019). IL-25 contributes to lung fibrosis by directly acting on alveolar epithelial cells and fibroblasts. Experimental Biology and Medicine, 244(9):770-80. https://doi.org/10.1177/1535370219843827
[44]Hams E, Armstrong ME, Barlow JL, Saunders SP, Schwartz C, Cooke G, et al. (2013). IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proceedings of the National Academy of Sciences, 111(1):367-72. https://doi.org/10.1073/pnas.1315854111
[45]Ritzmann F, Lunding LP, Bals R, Wegmann M, Beisswenger C. (2022). IL-17 Cytokines and Chronic Lung Diseases. Cells, 11(14). https://doi.org/10.3390/cells11142132
[46]Bellou V, Belbasis L, Evangelou E. (2021). Tobacco Smoking and Risk for Pulmonary Fibrosis. Ches, 160(3):983-93. https://doi.org/10.1016/j.chest.2021.04.035
[47]Sgalla G, Iovene B, Calvello M, Ori M, Varone F, Richeldi L. (2018). Idiopathic pulmonary fibrosis: pathogenesis and management. Respiratory Research,19(1). https://doi.org/10.1186/s12931-018-0730-2
[48]Li X, Bechara R, Zhao J, McGeachy MJ, Gaffen SL. (2019). IL-17 receptor–based signaling and implications for disease. Nature Immunology, 20(12):1594-602. https://doi.org/10.1038/s41590-019-0514-y
[49]McElroy AN, Invernizzi R, Laskowska JW, O'Neill A, Doroudian M, Moghoofei M, et al. (2022). Candidate Role for Toll-like Receptor 3 L412F Polymorphism and Infection in Acute Exacerbation of Idiopathic Pulmonary Fibrosis. American journal of respiratory and critical care medicine, 205(5), 550–562. https://doi.org/10.1164/rccm.202010-3880OC
[50]Theune WC, Chen J, Theune EV, Ye X, Ménoret A, Vella AT, et al. (2024). Interleukin-17 directly stimulates tumor infiltrating Tregs to prevent cancer development. Frontiers in Immunology, 15. https://doi.org/10.3389/fimmu.2024.1408710
[51]Li G, Chen H, Liu L, Xiao P, Xie Y, Geng X, et al. (2021). Role of Interleukin-17 in Acute Pancreatitis. Frontiers in Immunology,12. https://doi.org/10.3389/fimmu.2021.674803
[52]Zwicky P, Unger S, Becher B. (2020). Targeting interleukin-17 in chronic inflammatory disease: A clinical perspective. Journal of Experimental Medicine, 217(1).
[53]Ladjevac N, Milovanovic M, Jevtovic A, Arsenijevic D, Stojanovic B, Dimitrijevic Stojanovic M, et al. (2023). The Role of IL-17 in the Pathogenesis of Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences, 24(12). https://doi.org/10.3390/ijms24129874
[54]Hadian Y, Bagood MD, Dahle SE, Sood A, Isseroff RR. (2019). Interleukin-17: Potential Target for Chronic Wounds. Mediators of inflammation, 2019. https://doi.org/10.1155/2019/1297675
[55]Flemming A. (2023). Why do IL-17-targeted therapies have limited efficacy?. Nature Reviews Immunology, 23(9), 543-543. https://doi.org/10.1038/s41577-023-00930-5
[56]Mills KHG. (2022). IL-17 and IL-17-producing cells in protection versus pathology. Nature reviews. Immunology, 23(1), 38–54. https://doi.org/10.1038/s41577-022-00746-9
[57]Wang J, Wang C, Liu L, Hong S, Ru Y, Sun X, et al. (2023). Adverse events associated with anti-IL-17 agents for psoriasis and psoriatic arthritis: a systematic scoping review. Frontiers in Immunology,14. https://doi.org/10.3389/fimmu.2023.993057
[58]Luo Q, Liu Y, Shi K, Shen X, Yang Y, Liang X, et al. (2023). An autonomous activation of interleukin-17 receptor signaling sustains inflammation and promotes disease progression. Immunity, 56(9):2006-20.e6. https://doi.org/10.1016/j.immuni.2023.06.012
[59]Maslennikov R, Ivashkin V, Vasilieva E, Chipurik M, Semikova P, Semenets V, et al. (2021).Interleukin 17 antagonist netakimab is effective and safe in the new coronavirus infection (COVID-19). European Cytokine Network, 32(1):8-14. https://doi.org/10.1684/ecn.2021.0463
[60]Avdeev SN, Trushenko NV, Tsareva NA, Yaroshetskiy AI, Merzhoeva ZM, Nuralieva GS, et al. (2021). Anti-IL-17 monoclonal antibodies in hospitalized patients with severe COVID-19: A pilot study. Cytokine, 146. https://doi.org/10.1016/j.cyto.2021.155627
[61]Wei Q, Liao J, Jiang M, Liu J, Liang X, Nong G. (2021). Relationship between Th17-mediated immunity and airway inflammation in childhood neutrophilic asthma. Allergy, Asthma & Clinical Immunology, 17(1). https://doi.org/10.1186/s13223-020-00504-3
[62]Xie Y, Abel PW, Casale TB, Tu Y. (2022). TH17 cells and corticosteroid insensitivity in severe asthma. Journal of Allergy and Clinical Immunology, 149(2):467-79. https://doi.org/10.1016/j.jaci.2021.12.769
[63]Zhang B, Dömling A. (2022). Small molecule modulators of IL-17A/IL-17RA: a patent review (2013-2021). Expert Opinion on Therapeutic Patents, 32(11):1161-73. https://doi.org/10.1080/13543776.2022.2143264
Type
Published
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
Issue
Section
License
Copyright (c) 2025 Life Conflux

This work is licensed under a Creative Commons Attribution 4.0 International License.







