Exploring New Therapeutic Targets for Myasthenia Gravis: A Plasma and Cerebrospinal Fluid Proteomics Mendelian Randomization Study
DOI:
https://doi.org/10.71321/ybarkw62Keywords:
Myasthenia Gravis, Mendelian Randomization, Proteomics, Cerebrospinal Fluid Proteins, Colocalization, Therapeutic TargetAbstract
Background: Myasthenia Gravis (MG) is a chronic autoimmune neuromuscular disorder that severely impacts patients' quality of life. Identifying plasma and cerebrospinal fluid (CSF) proteins with a genetic causal relationship to MG may provide novel therapeutic targets.
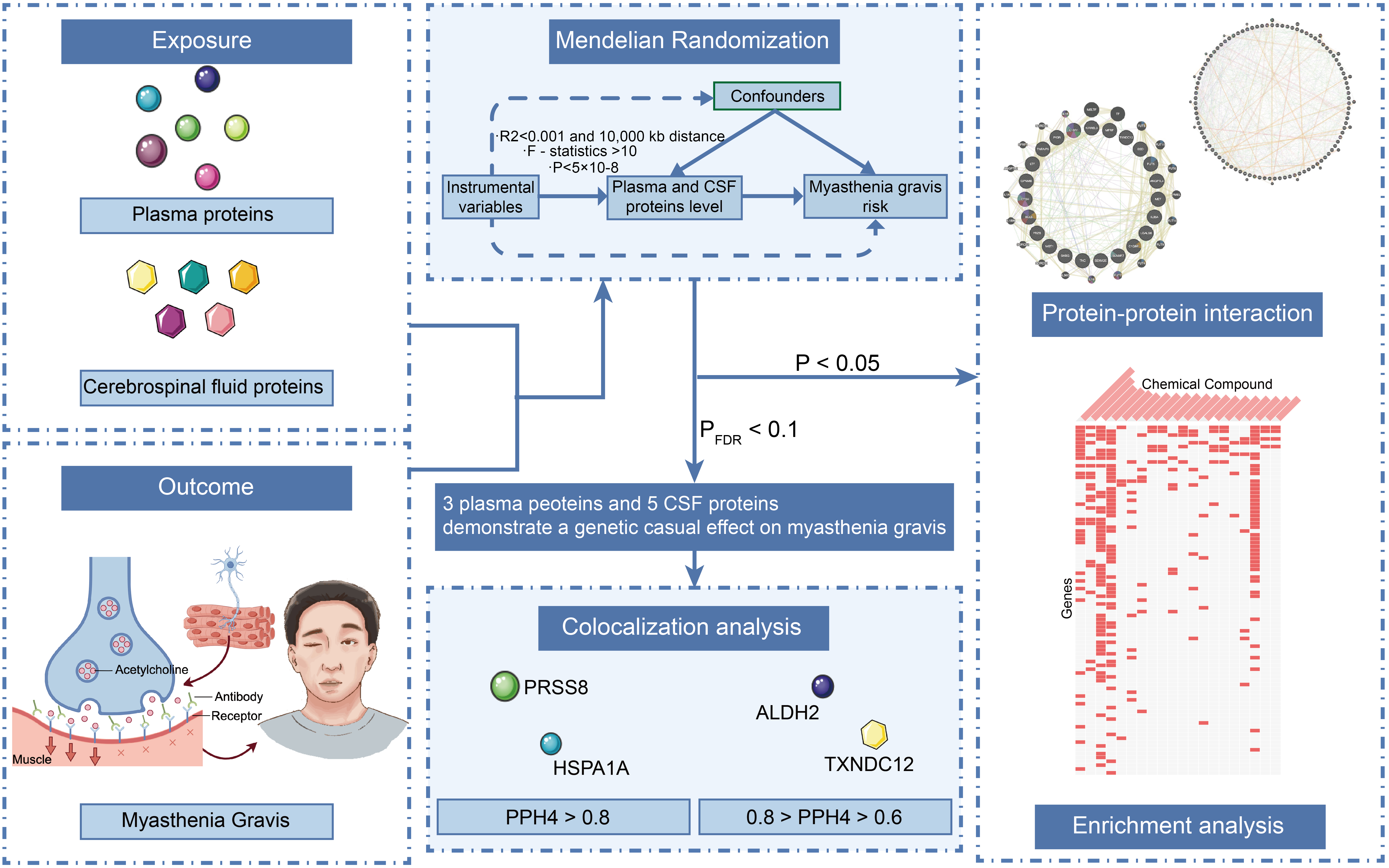
Methods: This study employed the Mendelian randomization (MR) approach, in combination with Bayesian colocalization analysis, to assess the causal relationship between 4,185 plasma proteins and 832 CSF proteins and the risk of MG. Sensitivity analyses were also performed to validate the robustness of the MR results. Additionally, protein–protein interaction networks and candidate drug predictions were utilized to elucidate the complex interactions between proteins and identify potential drug targets.
Results: Three plasma proteins and five CSF proteins were significantly associated with MG risk. ALDH2, HSPA1A, PRSS8, MFRP, CTSH, SHBG, and TXNDC12 were found to increase MG risk, while IL36A was negatively correlated. Further colocalization analysis revealed strong evidence for the associations between PRSS8 and HSPA1A with MG (pph4 > 0.8), and substantial evidence for TXNDC12 and ALDH2 (0.8 > pph4 > 0.6).
Conclusion: This study employed proteomics-based MR to identify several plasma and CSF proteins significantly associated with the risk of MG. Notably, PRSS8, HSPA1A, TXNDC12, and ALDH2 emerge as potential therapeutic targets for MG. While these findings offer valuable insights into the pathological mechanisms of MG and the development of novel therapeutic strategies, further research is required to evaluate the feasibility and clinical efficacy of these candidate proteins.
References
[1] Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, & Verschuuren J. (2019). Myasthenia gravis. Nat Rev Dis Primers, 5(1), 30. https://doi.org/10.1038/s41572-019-0079-y
[2] Moris G, Arboleya S, Mancabelli L, Milani C, Ventura M, de Los Reyes-Gavilán CG, et al. (2018). Fecal microbiota profile in a group of myasthenia gravis patients. Sci Rep, 8(1), 14384. https://doi.org/10.1038/s41598-018-32700-y
[3] Vincent A, Palace J, & Hilton-Jones D. (2001). Myasthenia gravis. Lancet, 357(9274), 2122-2128. https://doi.org/10.1016/s0140-6736(00)05186-2
[4] Iorio R. (2024). Myasthenia gravis: the changing treatment landscape in the era of molecular therapies. Nat Rev Neurol, 20(2), 84-98. https://doi.org/10.1038/s41582-023-00916-w
[5] Gilhus NE, & Verschuuren JJ. (2015). Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol, 14(10), 1023-1036. https://doi.org/10.1016/s1474-4422(15)00145-3
[6] Sieb JP. (2014). Myasthenia gravis: an update for the clinician. Clin Exp Immunol, 175(3), 408-418. https://doi.org/10.1111/cei.12217
[7] Alhaidar MK, Abumurad S, Soliven B, & Rezania K. (2022). Current Treatment of Myasthenia Gravis. J Clin Med, 11(6). https://doi.org/10.3390/jcm11061597
[8] Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo HC, Marx A, et al. (2016). Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med, 375(6), 511-522. https://doi.org/10.1056/NEJMoa1602489
[9] Narayanaswami P, Sanders DB, Wolfe G, Benatar M, Cea G, Evoli A, et al. (2021). International Consensus Guidance for Management of Myasthenia Gravis: 2020 Update. Neurology, 96(3), 114-122. https://doi.org/10.1212/wnl.0000000000011124
[10] Henry A, Gordillo-Marañón M, Finan C, Schmidt AF, Ferreira JP, Karra R, et al. (2022). Therapeutic Targets for Heart Failure Identified Using Proteomics and Mendelian Randomization. Circulation, 145(16), 1205-1217. https://doi.org/10.1161/circulationaha.121.056663
[11] Huang X, An X, Gao X, Wang N, Liu J, Zhang Y, et al. (2024). Serum amyloid A facilitates expansion of CD4(+) T cell and CD19(+) B cell subsets implicated in the severity of myasthenia gravis patients. J Neurochem, 168(3), 224-237. https://doi.org/10.1111/jnc.16047
[12] Roche JC, Capablo JL, Larrad L, Gervas-Arruga J, Ara JR, Sánchez A, et al. (2011). Increased serum interleukin-17 levels in patients with myasthenia gravis. Muscle Nerve, 44(2), 278-280. https://doi.org/10.1002/mus.22070
[13] Ma C, Liu D, Wang B, Yang Y, & Zhu R. (2024). Advancements and prospects of novel biologicals for myasthenia gravis: toward personalized treatment based on autoantibody specificities. Front Pharmacol, 15, 1370411. https://doi.org/10.3389/fphar.2024.1370411
[14] Finan C, Gaulton A, Kruger FA, Lumbers RT, Shah T, Engmann J, et al. (2017). The druggable genome and support for target identification and validation in drug development. Sci Transl Med, 9(383). https://doi.org/10.1126/scitranslmed.aag1166
[15] Saccà F, Barnett C, Vu T, Peric S, Phillips GA, Zhao S, et al. (2023). Efgartigimod improved health-related quality of life in generalized myasthenia gravis: results from a randomized, double-blind, placebo-controlled, phase 3 study (ADAPT). J Neurol, 270(4), 2096-2105. https://doi.org/10.1007/s00415-022-11517-w
[16] Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafò MR, et al. (2022). Mendelian randomization. Nat Rev Methods Primers, 2. https://doi.org/10.1038/s43586-021-00092-5
[17] Pietzner M, Wheeler E, Carrasco-Zanini J, Cortes A, Koprulu M, Wörheide MA, et al. (2021). Mapping the proteo-genomic convergence of human diseases. Science, 374(6569), eabj1541. https://doi.org/10.1126/science.abj1541
[18] Yang C, Farias FHG, Ibanez L, Suhy A, Sadler B, Fernandez MV, et al. (2021). Genomic atlas of the proteome from brain, CSF and plasma prioritizes proteins implicated in neurological disorders. Nat Neurosci, 24(9), 1302-1312. https://doi.org/10.1038/s41593-021-00886-6
[19] Chia R, Saez-Atienzar S, Murphy N, Chiò A, Blauwendraat C, Roda RH, et al. (2022). Identification of genetic risk loci and prioritization of genes and pathways for myasthenia gravis: a genome-wide association study. Proc Natl Acad Sci U S A, 119(5). https://doi.org/10.1073/pnas.2108672119
[20] Burgess S, Butterworth A, & Thompson SG. (2013). Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol, 37(7), 658-665. https://doi.org/10.1002/gepi.21758
[21] Zhao H, Zhou Y, Wang Z, Zhang X, Chen L, & Hong Z. (2024). Plasma proteins and psoriatic arthritis: a proteome-wide Mendelian randomization study. Front Immunol, 15, 1417564. https://doi.org/10.3389/fimmu.2024.1417564
[22] Morin PA, Martien KK, & Taylor BL. (2009). Assessing statistical power of SNPs for population structure and conservation studies. Mol Ecol Resour, 9(1), 66-73. https://doi.org/10.1111/j.1755-0998.2008.02392.x
[23] Lutz SM, Voorhies K, Wu AC, Hokanson J, Vansteelandt S, & Lange C. (2022). The influence of unmeasured confounding on the MR Steiger approach. Genet Epidemiol, 46(2), 139-141. https://doi.org/10.1002/gepi.22442
[24] Giambartolomei C, Vukcevic D, Schadt EE, Franke L, Hingorani AD, Wallace C, et al. (2014). Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet, 10(5), e1004383. https://doi.org/10.1371/journal.pgen.1004383
[25] Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, et al. (2010). The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res, 38(Web Server issue), W214-220. https://doi.org/10.1093/nar/gkq537
[26] Santos R, Ursu O, Gaulton A, Bento AP, Donadi RS, Bologa CG, et al. (2017). A comprehensive map of molecular drug targets. Nat Rev Drug Discov, 16(1), 19-34. https://doi.org/10.1038/nrd.2016.230
[27] Yoo M, Shin J, Kim J, Ryall KA, Lee K, Lee S, et al. (2015). DSigDB: drug signatures database for gene set analysis. Bioinformatics, 31(18), 3069-3071. https://doi.org/10.1093/bioinformatics/btv313
[28] Wallace C. (2021). A more accurate method for colocalisation analysis allowing for multiple causal variants. PLoS Genet, 17(9), e1009440. https://doi.org/10.1371/journal.pgen.1009440
[29] Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, et al. (2016). Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res, 44(W1), W90-97. https://doi.org/10.1093/nar/gkw377
[30] Szabo R, Uzzun Sales K, Kosa P, Shylo NA, Godiksen S, Hansen KK, et al. (2012). Reduced prostasin (CAP1/PRSS8) activity eliminates HAI-1 and HAI-2 deficiency-associated developmental defects by preventing matriptase activation. PLoS Genet, 8(8), e1002937. https://doi.org/10.1371/journal.pgen.1002937
[31] Martin CE, & List K. (2019). Cell surface-anchored serine proteases in cancer progression and metastasis. Cancer Metastasis Rev, 38(3), 357-387. https://doi.org/10.1007/s10555-019-09811-7
[32] Uchimura K, Hayata M, Mizumoto T, Miyasato Y, Kakizoe Y, Morinaga J, et al. (2014). The serine protease prostasin regulates hepatic insulin sensitivity by modulating TLR4 signalling. Nat Commun, 5, 3428. https://doi.org/10.1038/ncomms4428
[33] Chen LM, Hatfield ML, Fu YY, & Chai KX. (2009). Prostasin regulates iNOS and cyclin D1 expression by modulating protease-activated receptor-2 signaling in prostate epithelial cells. Prostate, 69(16), 1790-1801. https://doi.org/10.1002/pros.21030
[34] Tüzün E, Huda R, & Christadoss P. (2011). Complement and cytokine based therapeutic strategies in myasthenia gravis. J Autoimmun, 37(2), 136-143. https://doi.org/10.1016/j.jaut.2011.05.006
[35] Nong W, Huang F, Mao F, Lao D, Gong Z, & Huang W. (2022). DCAF12 and HSPA1A May Serve as Potential Diagnostic Biomarkers for Myasthenia Gravis. Biomed Res Int, 2022, 8587273. https://doi.org/10.1155/2022/8587273
[36] Mayer MP, & Bukau B. (2005). Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci, 62(6), 670-684. https://doi.org/10.1007/s00018-004-4464-6
[37] Srivastava P. (2002). Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol, 2(3), 185-194. https://doi.org/10.1038/nri749
[38] Basu S, Binder RJ, Suto R, Anderson KM, & Srivastava PK. (2000). Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol, 12(11), 1539-1546. https://doi.org/10.1093/intimm/12.11.1539
[39] Yuan K, Xie K, Lan T, Xu L, Chen X, Li X, et al. (2020). TXNDC12 promotes EMT and metastasis of hepatocellular carcinoma cells via activation of β-catenin. Cell Death Differ, 27(4), 1355-1368. https://doi.org/10.1038/s41418-019-0421-7
[40] Malhotra JD, & Kaufman RJ. (2007). The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol, 18(6), 716-731. https://doi.org/10.1016/j.semcdb.2007.09.003
[41] Ohta S, Ohsawa I, Kamino K, Ando F, & Shimokata H. (2004). Mitochondrial ALDH2 deficiency as an oxidative stress. Ann N Y Acad Sci, 1011, 36-44. https://doi.org/10.1007/978-3-662-41088-2_4
[42] Wei T, Zhu Z, Liu L, Liu B, Wu M, Zhang W, et al. (2023). Circulating levels of cytokines and risk of cardiovascular disease: a Mendelian randomization study. Front Immunol, 14, 1175421. https://doi.org/10.3389/fimmu.2023.1175421
Type
Published
Data Availability Statement
The data that supports the findings of this study are available in the supplementary materials of this article.
Issue
Section
License
Copyright (c) 2025 Life Conflux

This work is licensed under a Creative Commons Attribution 4.0 International License.







