Causal Relationship between Chronic Gastritis and Sleep Apnea Syndrome: A Bidirectional and Two-step Mendelian Randomization Study
DOI:
https://doi.org/10.71321/zrtsfk74Keywords:
sleep apnea syndrome, chronic gastritis, a two-step Mendelian Randomization Study, a two-sample Mendelian randomizationAbstract
Background: Sleep apnea syndrome (SAS) and chronic gastritis are prevalent in middle-aged and elderly people. Although clinical observations suggest an association between the two diseases, the causal relationship between them has not been clarified. This study aims to explore the causal relationship between SAS and chronic gastritis and to elucidate the possible mediating mechanisms.
Methods: The causal relationship between SAS and chronic gastritis was assessed using five methods, primarily utilizing the Inverse Variance Weighted (IVW) approach, with other methods categorized as sensitivity analyses. A two-step Mendelian randomization study was conducted using a genome-wide association study (GWAS) dataset to evaluate the mediating role of signatures in immune cell and inflammatory proteins. Sensitivity analyses were conducted to determine the robustness of the results. To systematically address horizontal pleiotropy, we employed the CAUSE framework, which distinguishes causal effects from confounding by comparing genetic architectures under competing causal and shared confounding models, while accounting for both linkage disequilibrium-related and independent pleiotropic pathways. The STROBE-MR guidelines were followed for MR results reporting.
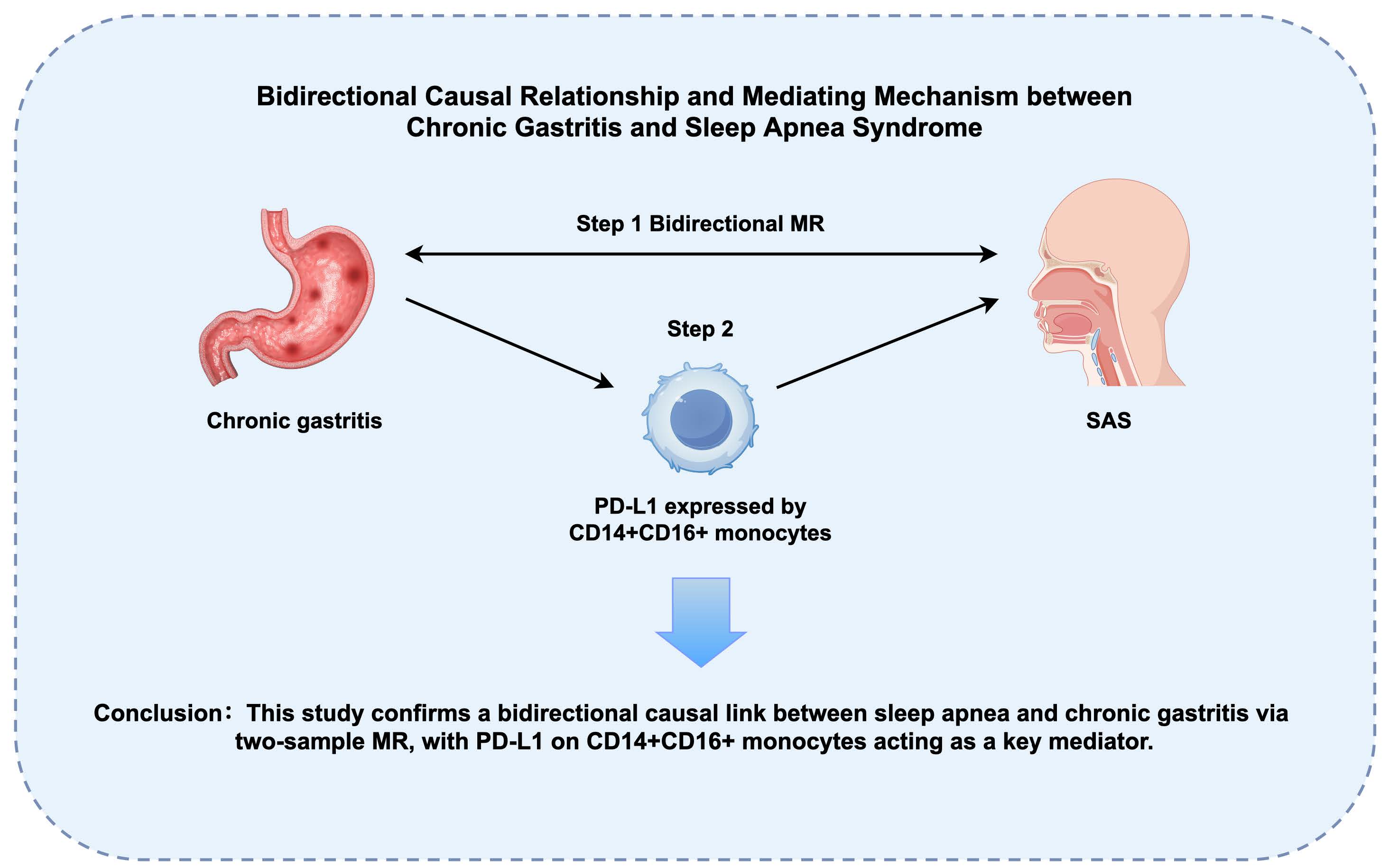
Results: Through two-sample MR analysis, we identified significant bidirectional causal relationships between sleep apnea syndrome (SAS) and chronic gastritis (forward: OR = 1.131, 95% CI: 1.031-1.240, p = 0.009; reverse: OR = 1.065, 95% CI: 1.007-1.128, p = 0.028). Simulation studies confirmed CAUSE's superior specificity in controlling false positives through its dual-model framework that explicitly accounts for pleiotropic pathways. Two-step MR analysis revealed that the changes in the level of PD-L1 expressed by CD14+CD16+ monocytes played a significant mediating role in the effect of chronic gastritis on SAS.
Conclusion: The bidirectional causal relationship between SAS and chronic gastritis was confirmed through two-sample and two-step MR analyses. Chronic gastritis may increase the risk of SAS through genetic signatures in immune cells, providing new perspectives for future research and aiding in the development of new prevention and treatment strategies.
References
[1] Wanyan, P., et al., Obstructive sleep apnea hypopnea syndrome: Protocol for the development of a core outcome set. Medicine (Baltimore), 2020. 99(34): p. e21591. DOI: https://doi.org/10.1097/md.0000000000021591.
[2] Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep, 1999. 22(5): p. 667-89.
[3] Piccirillo, F., et al., A State-of-the-Art Review on Sleep Apnea Syndrome and Heart Failure. Am J Cardiol, 2023. 195: p. 57-69. DOI: https://doi.org/10.1016/j.amjcard.2023.02.020.
[4] Peppard, P.E., et al., Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol, 2013. 177(9): p. 1006-14. DOI: https://doi.org/10.1093/aje/kws342.
[5] Guidelines for diagnosis and treatment of chronic gastritis in China (2022, Shanghai). J Dig Dis, 2023. 24(3): p. 150-180. DOI: https://doi.org/10.1111/1751-2980.13193.
[6] Du, Y., et al., Chronic gastritis in China: a national multi-center survey. BMC Gastroenterol, 2014. 14: p. 21. DOI: https://doi.org/10.1186/1471-230x-14-21.
[7] Sipponen, P. and H.I. Maaroos, Chronic gastritis. Scand J Gastroenterol, 2015. 50(6): p. 657-67. DOI: https://doi.org/10.3109/00365521.2015.1019918.
[8] Orr, W.C., et al., The effect of sleep on gastrointestinal functioning in common digestive diseases. Lancet Gastroenterol Hepatol, 2020. 5(6): p. 616-624. DOI: https://doi.org/10.1016/s2468-1253(19)30412-1.
[9] Vege, S.S., et al., Functional gastrointestinal disorders among people with sleep disturbances: a population-based study. Mayo Clin Proc, 2004. 79(12): p. 1501-6. DOI: https://doi.org/10.4065/79.12.1501.
[10] Zhang, Z., et al., Investigating the causal links between obstructive sleep apnea and gastrointestinal diseases mediated by metabolic syndrome through mendelian randomization. Sci Rep, 2024. 14(1): p. 26247. DOI: https://doi.org/10.1038/s41598-024-77471-x.
[11] Xia, W., et al., Relationship between obstructive sleep apnoea syndrome and essential hypertension: a dose-response meta-analysis. Sleep Med, 2018. 47: p. 11-18. DOI: https://doi.org/10.1016/j.sleep.2018.03.016.
[12] Lawlor, D.A., et al., Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med, 2008. 27(8): p. 1133-63. DOI: https://doi.org/10.1002/sim.3034.
[13] Mashaqi, S. and D. Gozal, Obstructive Sleep Apnea and Systemic Hypertension: Gut Dysbiosis as the Mediator? J Clin Sleep Med, 2019. 15(10): p. 1517-1527. DOI: https://doi.org/10.5664/jcsm.7990.
[14] Lu, D., et al., Profiling of lung microbiota in the patients with obstructive sleep apnea. Medicine (Baltimore), 2018. 97(26): p. e11175. DOI: https://doi.org/10.1097/md.0000000000011175.
[15] Sun, M., et al., Causal relationships of Helicobacter pylori and related gastrointestinal diseases on Type 2 diabetes: Univariable and Multivariable Mendelian randomization. PLoS One, 2024. 19(4): p. e0300835. DOI: https://doi.org/10.1371/journal.pone.0300835.
[16] Li, W., et al., Complex causal association between genetically predicted 731 immunocyte phenotype and osteonecrosis: a bidirectional two-sample Mendelian randomization analysis. Int J Surg, 2024. 110(6): p. 3285-3293. DOI: https://doi.org/10.1097/js9.0000000000001327.
[17] Xiao, C., et al., Two-sample Mendelian randomization analysis of 91 circulating inflammatory protein levels and amyotrophic lateral sclerosis. Front Aging Neurosci, 2024. 16: p. 1367106. DOI: https://doi.org/10.3389/fnagi.2024.1367106.
[18] Burgess, S., et al., Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res, 2019. 4: p. 186. DOI: https://doi.org/10.12688/wellcomeopenres.15555.3.
[19] Davey Smith, G. and G. Hemani, Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet, 2014. 23(R1): p. R89-98. DOI: https://doi.org/10.1093/hmg/ddu328.
[20] Verbanck, M., et al., Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet, 2018. 50(5): p. 693-698. DOI: https://doi.org/10.1038/s41588-018-0099-7.
[21] Bowden, J., G. Davey Smith, and S. Burgess, Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol, 2015. 44(2): p. 512-25. DOI: https://doi.org/10.1093/ije/dyv080.
[22] Mounier, N. and Z. Kutalik, Bias correction for inverse variance weighting Mendelian randomization. Genet Epidemiol, 2023. 47(4): p. 314-331. DOI: https://doi.org/10.1002/gepi.22522.
[23] Morrison, J., et al., Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nat Genet, 2020. 52(7): p. 740-747. DOI: https://doi.org/10.1038/s41588-020-0631-4.
[24] Skrivankova, V.W., et al., Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. Bmj, 2021. 375: p. n2233. DOI: https://doi.org/10.1136/bmj.n2233.
[25] Carter, A.R., et al., Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol, 2021. 36(5): p. 465-478. DOI: https://doi.org/10.1007/s10654-021-00757-1.
[26] Hemani, G., J. Bowden, and G. Davey Smith, Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet, 2018. 27(R2): p. R195-r208. DOI: https://doi.org/10.1093/hmg/ddy163.
[27] Bowden, J., et al., Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol, 2016. 40(4): p. 304-14. DOI: https://doi.org/10.1002/gepi.21965.
[28] Hu, J., et al., Reverse causal relationship between periodontitis and shortened telomere length: Bidirectional two-sample Mendelian random analysis. Front Immunol, 2022. 13: p. 1057602. DOI: https://doi.org/10.3389/fimmu.2022.1057602.
[29] Burgess, S. and S.G. Thompson, Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol, 2017. 32(5): p. 377-389. DOI: https://doi.org/10.1007/s10654-017-0255-x.
[30] Hemani, G., et al., The MR-Base platform supports systematic causal inference across the human phenome. Elife, 2018. 7. DOI: https://doi.org/10.7554/eLife.34408.
[31] Larsson, S.C., et al., Genetic predisposition to smoking in relation to 14 cardiovascular diseases. Eur Heart J, 2020. 41(35): p. 3304-3310. DOI: https://doi.org/10.1093/eurheartj/ehaa193.
[32] Kurki, M.I., et al., FinnGen provides genetic insights from a well-phenotyped isolated population. Nature, 2023. 613(7944): p. 508-518. DOI: https://doi.org/10.1038/s41586-022-05473-8.
[33] Yan, W., et al., Obstructive sleep apnea and 19 gastrointestinal diseases: a Mendelian randomization study. Front Psychiatry, 2024. 15: p. 1256116. DOI: https://doi.org/10.3389/fpsyt.2024.1256116.
[34] Ma, B., et al., Association Between Abdominal Adipose Tissue Distribution and Obstructive Sleep Apnea in Chinese Obese Patients. Front Endocrinol (Lausanne), 2022. 13: p. 847324. DOI: https://doi.org/10.3389/fendo.2022.847324.
[35] Antonaglia, C., et al., Low arousal threshold: a common pathophysiological trait in patients with obstructive sleep apnea syndrome and asthma. Sleep Breath, 2023. 27(3): p. 933-941. DOI: https://doi.org/10.1007/s11325-022-02665-4.
[36] Hatamnejad, M.R., et al., Selective serotonin reuptake inhibitors and inflammatory bowel disease; Beneficial or malpractice. Front Immunol, 2022. 13: p. 980189. DOI: https://doi.org/10.3389/fimmu.2022.980189.
[37] Wu, J., et al., Disrupted intestinal structure in a rat model of intermittent hypoxia. Mol Med Rep, 2016. 13(5): p. 4407-13. DOI: https://doi.org/10.3892/mmr.2016.5068.
[38] Gold, A.R. and M.S. Gold, Con: Sleep fragmentation causes hypersomnolence in OSA. Sleep Med Rev, 2021. 55: p. 101399. DOI: https://doi.org/10.1016/j.smrv.2020.101399.
[39] Liu, H., et al., Effect of Helicobacter pylori-Associated Chronic Gastritis on Autonomous Activity and Sleep Quality in Mice. Front Pharmacol, 2022. 13: p. 785105. DOI: https://doi.org/10.3389/fphar.2022.785105.
[40] Chiba, S., 0700 Prediction Of Surgical Outcome Using Respiratory Pattern Clafification. Sleep, 2020. 43(Supplement_1): p. A267-A267. DOI: https://doi.org/10.1093/sleep/zsaa056.696 %J Sleep.
[41] Mya, H.T., et al., PD-1 and PD-L1 Are Overexpressed in the "Intermediate CD14+CD16+" and "Non Classical CD14lowCD16+" but Not in the "Classical CD14+CD16-" Monocytes in the Peripheral Blood of Chronic Myelomonocytic Leukemia. Blood, 2015. 126(23): p. 1694-1694. DOI: https://doi.org/10.1182/blood.V126.23.1694.1694 %J Blood.
[42] Bolasco, P., et al., Could there be Haemodynamic Stress Effects on Pro-Inflammatory CD14+CD16+ Monocytes during Convective-Diffusive Treatments? A Prospective Randomized Controlled Trial. Blood Purif, 2019. 47(4): p. 385-394. DOI: https://doi.org/10.1159/000494711.
[43] Ziegler-Heitbrock, L., The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol, 2007. 81(3): p. 584-92. DOI: https://doi.org/10.1189/jlb.0806510.
[44] Costa, F., et al., PD-L1/PD-1 Pattern of Expression Within the Bone Marrow Immune Microenvironment in Smoldering Myeloma and Active Multiple Myeloma Patients. Front Immunol, 2020. 11: p. 613007. DOI: https://doi.org/10.3389/fimmu.2020.613007.
[45] Johnson, M.D., et al., Model-based analysis of implanted hypoglossal nerve stimulation for the treatment of obstructive sleep apnea. Sleep, 2021. 44(44 Suppl 1): p. S11-s19. DOI: https://doi.org/10.1093/sleep/zsaa269.
[46] Go, D.M., et al., Programmed Death Ligand 1-Expressing Classical Dendritic Cells MitigateHelicobacter-Induced Gastritis. Cell Mol Gastroenterol Hepatol, 2021. 12(2): p. 715-739. DOI: https://doi.org/10.1016/j.jcmgh.2021.04.007.
Type
Published
Data Availability Statement
This study analyzed datasets that are publicly accessible. These datasets can be found at the following URLs: FinnGen (https://storage.googleapis.com/finngen-public-data-r9/summary_stats/finngen_R9_M13_OSTEONECROSIS.gz) and GWAS Catalog (https://www.ebi.ac.uk/gwas/downloads/summary-statistics).
Issue
Section
License
Copyright (c) 2025 Life Conflux

This work is licensed under a Creative Commons Attribution 4.0 International License.







