Construction of prognostic model and tumor microenvironment landscape based on cuproptosis-related subtypes in melanoma
DOI:
https://doi.org/10.71321/vxy0xd87Keywords:
melanoma, cuproptosis, tumor microenvironment, differentially expressed genes, risk score, bioinformatics analysisAbstract
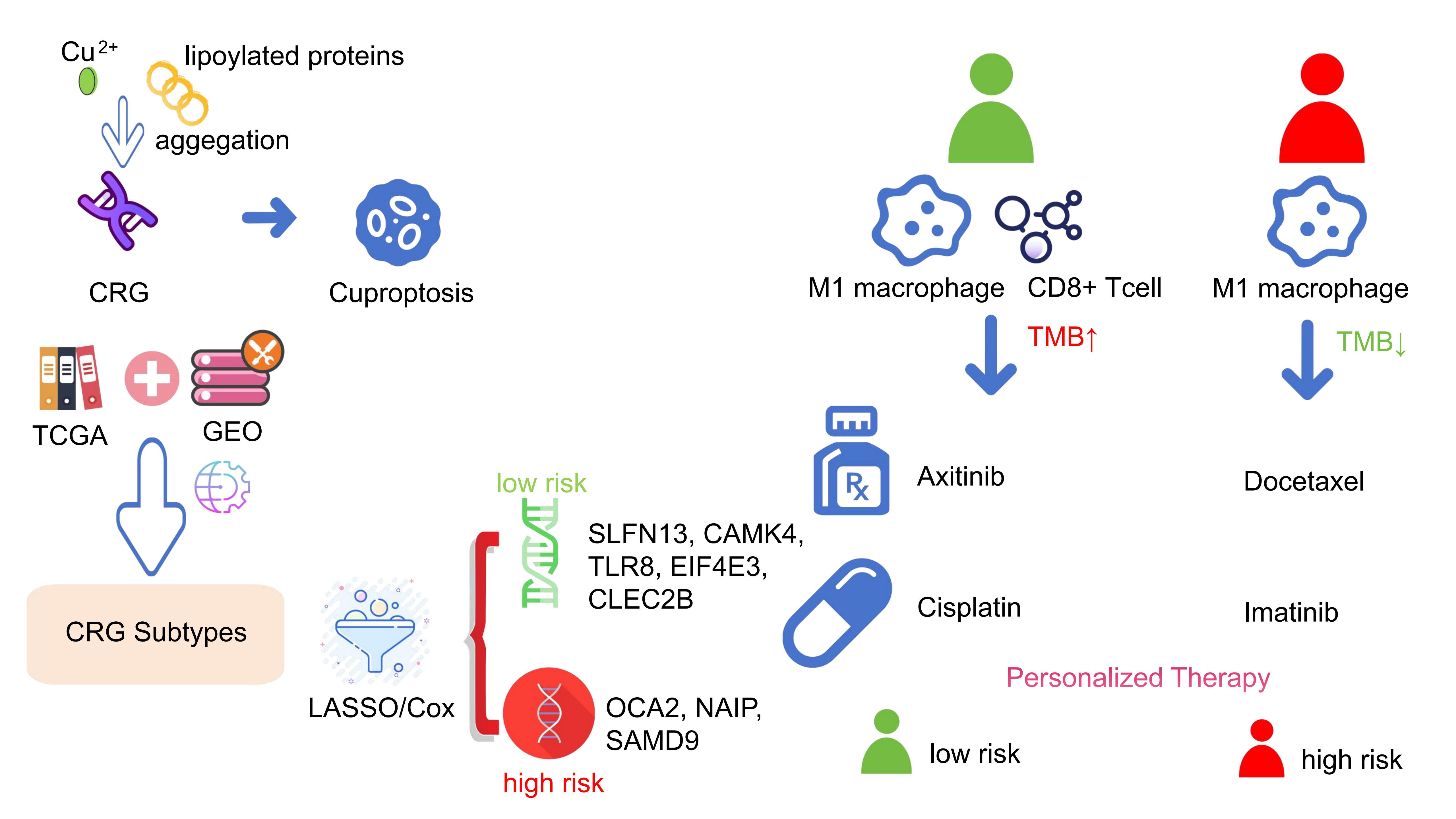
Background: Melanoma, known for its aggressive nature and poor prognosis, may be impacted by cuproptosis, a recently discovered form of programmed cell death. Despite its unclear mechanisms, preliminary studies suggested a link between cuproptosis and cancer progression and metastasis. We aimed to investigate the association between cuproptosis-related genes (CRGs) and melanoma to enhance prognostic and therapeutic strategies.
Method: In this study, we downloaded transcriptome RNA-seqs and clinical information of all melanoma patients from The Cancer Genome Atlas (TCGA) database, selected a dataset from Gene Expression Omnibus (GEO) databases, and merged the two datasets. After univariate regression analysis, all the samples were categorized into three groups based on expression levels of CRGs. Differential expression analysis was carried out for three CRG clusters to obtain the significant differentially expressed genes (DEGs). After univariate Cox regression analysis, multivariate Cox regression analysis and the least absolute shrinkage and selection operator (LASSO) algorithm were performed on DEGs, the prognosis related genes were screened to establish a prognosis prediction model. The model's accuracy was validated through Kaplan-Meier analysis, receiver operating characteristic (ROC) curve, nomogram, and independent prognostic analysis. Additionally, we compared the immune scores of the tumor microenvironment, tumor mutation burden, tumor immune dysfunction and exclusion, and drug sensitivity between high-risk and low-risk groups.
Results: Through algorithm analysis, eight genes significantly related to prognosis were identified, among which SLFN13, CAMK4, TLR8, EIF4E3, and CLEC2B were low-risk genes, OCA2, NAIP, and SAMD9 were high-risk genes. Using these genes, we established a prognostic model that effectively distinguishes between different survival outcomes, with the low-risk group showing a markedly higher long-term survival rate.
Conclusion: In conclusion, based on the research of cuproptosis subtypes, we identify the DEG with predictive potential and establish a prognosis prediction model. This study may provide a reference for the prognosis and clinical treatment of melanoma patients from the perspective of cuproptosis.
References
[1] Shain AH, & Bastian BC. (2020). Author Correction: From melanocytes to melanomas. Nature Reviews Cancer, 20(6), 355-355. https://doi.org/10.1038/s41568-020-0269-7
[2] Long GV, Swetter SM, Menzies AM, Gershenwald JE, & Scolyer RA. (2023). Cutaneous melanoma. The Lancet, 402(10400), 485-502. https://doi.org/10.1016/S0140-6736(23)00821-8
[3] Schadendorf D, Fisher DE, Garbe C, Gershenwald JE, Grob J-J, Halpern A, et al. (2015). Melanoma. Nature Reviews Disease Primers, 1(1), 15003. https://doi.org/10.1038/nrdp.2015.3
[4] Corneli P, Zalaudek I, Magaton Rizzi G, & di Meo N. (2018). Improving the early diagnosis of early nodular melanoma: can we do better? Expert Review of Anticancer Therapy, 18(10), 1007-1012. https://doi.org/10.1080/14737140.2018.1507822
[5] Cullen JK, Simmons JL, Parsons PG, & Boyle GM. (2020). Topical treatments for skin cancer. Advanced Drug Delivery Reviews, 153, 54-64. https://doi.org/10.1016/j.addr.2019.11.002
[6] Leonardi, Falzone, Salemi, Zanghì, Spandidos, McCubrey, et al. (2018). Cutaneous melanoma: From pathogenesis to therapy (Review). International Journal of Oncology, 52(4), 1071-1080. https://doi.org/10.3892/ijo.2018.4287
[7] Pavri SN, Clune J, Ariyan S, & Narayan D. (2016). Malignant Melanoma: Beyond the Basics. Plastic and Reconstructive Surgery, 138(2), 330e-340e. https://doi.org/10.1097/prs.0000000000002367
[8] Parra LM, & Webster RM. (2022). The malignant melanoma market. Nat Rev Drug Discov, 21(7), 489-490. https://doi.org/10.1038/d41573-022-00075-5
[9] Jenkins RW, & Fisher DE. (2021). Treatment of Advanced Melanoma in 2020 and Beyond. Journal of Investigative Dermatology, 141(1), 23-31. https://doi.org/10.1016/j.jid.2020.03.943
[10] Guo W, Wang H, & Li C. (2021). Signal pathways of melanoma and targeted therapy. Signal Transduction and Targeted Therapy, 6(1), 424. https://doi.org/10.1038/s41392-021-00827-6
[11] Winder, M., Virós, A. (2017). Mechanisms of Drug Resistance in Melanoma. In: Mandalà, M., Romano, E. (eds) Mechanisms of Drug Resistance in Cancer Therapy. Handbook of Experimental Pharmacology, vol 249. Springer, Cham. https://doi.org/10.1007/164_2017_17
[12] Witt B, Schaumlöffel D, & Schwerdtle T. (2020). Subcellular Localization of Copper—Cellular Bioimaging with Focus on Neurological Disorders. International Journal of Molecular Sciences, 21(7).
[13] Ge EJ, Bush AI, Casini A, Cobine PA, Cross JR, DeNicola GM, et al. (2022). Connecting copper and cancer: from transition metal signalling to metalloplasia. Nature Reviews Cancer, 22(2), 102-113. https://doi.org/10.1038/s41568-021-00417-2
[14] Wazir SM, & Ghobrial I. (2017). Copper deficiency, a new triad: anemia, leucopenia, and myeloneuropathy. Journal of Community Hospital Internal Medicine Perspectives, 7(4), 265-268. https://doi.org/10.1080/20009666.2017.1351289
[15] Scheiber I, Dringen R, & Mercer JFB. (2013). Copper: Effects of Deficiency and Overload. In A. Sigel, H. Sigel, & R. K. O. Sigel (Eds.), Interrelations between Essential Metal Ions and Human Diseases (10.1007/978-94-007-7500-8_11pp. 359-387). Springer Netherlands. https://doi.org/10.1007/978-94-007-7500-8_11
[16] Aspli KT, Flaten TP, Roos PM, Holmøy T, Skogholt JH, & Aaseth J. (2015). Iron and copper in progressive demyelination – New lessons from Skogholt's disease. Journal of Trace Elements in Medicine and Biology, 31, 183-187. https://doi.org/https://doi.org/10.1016/j.jtemb.2014.12.002
[17] Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, et al. (2022). Copper induces cell death by targeting lipoylated TCA cycle proteins. Science, 375(6586), 1254-1261. https://doi.org/doi:10.1126/science.abf0529
[18] Blockhuys S, Celauro E, Hildesjö C, Feizi A, Stål O, Fierro-González JC, et al. (2016). Defining the human copper proteome and analysis of its expression variation in cancers†. Metallomics, 9(2), 112-123. https://doi.org/10.1039/c6mt00202a
[19] Ackerman CM, Lee S, & Chang CJ. (2017). Analytical Methods for Imaging Metals in Biology: From Transition Metal Metabolism to Transition Metal Signaling. Analytical Chemistry, 89(1), 22-41. https://doi.org/10.1021/acs.analchem.6b04631
[20] Wang Y, Zhang L, & Zhou F. (2022). Cuproptosis: a new form of programmed cell death. Cellular & Molecular Immunology, 19(8), 867-868. https://doi.org/10.1038/s41423-022-00866-1
[21] Kim B-E, Nevitt T, & Thiele DJ. (2008). Mechanisms for copper acquisition, distribution and regulation. Nature Chemical Biology, 4(3), 176-185. https://doi.org/10.1038/nchembio.72
[22] Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, et al. (2019). Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Annals of Oncology, 30(1), 44-56. https://doi.org/10.1093/annonc/mdy495
[23] Zhu Y, Yao S, & Chen L. (2011). Cell Surface Signaling Molecules in the Control of Immune Responses: A Tide Model. Immunity, 34(4), 466-478. https://doi.org/https://doi.org/10.1016/j.immuni.2011.04.008
[24] Auslander N, Zhang G, Lee JS, Frederick DT, Miao B, Moll T, et al. (2018). Robust prediction of response to immune checkpoint blockade therapy in metastatic melanoma. Nature Medicine, 24(10), 1545-1549. https://doi.org/10.1038/s41591-018-0157-9
[25] McGrail DJ, Pilié PG, Rashid NU, Voorwerk L, Slagter M, Kok M, et al. (2021). High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Annals of Oncology, 32(5), 661-672. https://doi.org/10.1016/j.annonc.2021.02.006
[26] Barroso-Sousa R, Jain E, Cohen O, Kim D, Buendia-Buendia J, Winer E, et al. (2020). Prevalence and mutational determinants of high tumor mutation burden in breast cancer. Annals of Oncology, 31(3), 387-394. https://doi.org/10.1016/j.annonc.2019.11.010
[27] Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, et al. (2017). Whole-genome landscapes of major melanoma subtypes. Nature, 545(7653), 175-180. https://doi.org/10.1038/nature22071
[28] Nikolaou V, & Stratigos AJ. (2014). Emerging trends in the epidemiology of melanoma. Br J Dermatol, 170(1), 11-19. https://doi.org/10.1111/bjd.12492
[29] Lin WM, & Fisher DE. (2017). Signaling and Immune Regulation in Melanoma Development and Responses to Therapy. Annual Review of Pathology: Mechanisms of Disease, 12(Volume 12, 2017), 75-102. https://doi.org/https://doi.org/10.1146/annurev-pathol-052016-100208
[30] Sun J, Carr MJ, & Khushalani NI. (2020). Principles of Targeted Therapy for Melanoma. Surgical Clinics of North America, 100(1), 175-188. https://doi.org/https://doi.org/10.1016/j.suc.2019.09.013
[31] Renz PF, Ghoshdastider U, Baghai Sain S, Valdivia-Francia F, Khandekar A, Ormiston M, et al. (2024). In vivo single-cell CRISPR uncovers distinct TNF programmes in tumour evolution. Nature, 632(8024), 419-428. https://doi.org/10.1038/s41586-024-07663-y
[32] da Silva DA, De Luca A, Squitti R, Rongioletti M, Rossi L, Machado CML, et al. (2022). Copper in tumors and the use of copper-based compounds in cancer treatment. Journal of Inorganic Biochemistry, 226, 111634. https://doi.org/https://doi.org/10.1016/j.jinorgbio.2021.111634
[33] Tang D, Kang R, Berghe TV, Vandenabeele P, & Kroemer G. (2019). The molecular machinery of regulated cell death. Cell Research, 29(5), 347-364. https://doi.org/10.1038/s41422-019-0164-5
[34] Lin Z, Xu Q, Miao D, & Yu F. (2021). An Inflammatory Response-Related Gene Signature Can Impact the Immune Status and Predict the Prognosis of Hepatocellular Carcinoma [Original Research]. Frontiers in Oncology, Volume 11 - 2021. https://doi.org/10.3389/fonc.2021.644416
[35] Liu H, Gao L, Li J, Zhai T, Xie T, & Xu Y. (2020). Identification and validation of a ferroptosis-related genes based prognostic signature for prostate cancer. bioRxiv.
[36] Sun J, Yue W, You J, Wei X, Huang Y, Ling Z, et al. (2021). Identification of a Novel Ferroptosis-Related Gene Prognostic Signature in Bladder Cancer [Original Research]. Frontiers in Oncology, Volume 11 - 2021. https://doi.org/10.3389/fonc.2021.730716
[37] Li Z, Lu J, Zeng G, Pang J, Zheng X, Feng J, et al. (2019). MiR-129-5p inhibits liver cancer growth by targeting calcium calmodulin-dependent protein kinase IV (CAMK4). Cell Death & Disease, 10(11), 789. https://doi.org/10.1038/s41419-019-1923-4
[38] Frega G, Wu Q, Le Naour J, Vacchelli E, Galluzzi L, Kroemer G, et al. (2020). Trial Watch: experimental TLR7/TLR8 agonists for oncological indications. OncoImmunology, 9(1), 1796002. https://doi.org/10.1080/2162402X.2020.1796002
[39] Osborne MJ, Volpon L, Kornblatt JA, Culjkovic-Kraljacic B, Baguet A, & Borden KLB. (2013). eIF4E3 acts as a tumor suppressor by utilizing an atypical mode of methyl-7-guanosine cap recognition. Proceedings of the National Academy of Sciences, 110(10), 3877-3882. https://doi.org/doi:10.1073/pnas.1216862110
[40] Li X, Tao X, & Ding X. (2022). An integrative analysis to reveal that CLEC2B and ferroptosis may bridge the gap between psoriatic arthritis and cancer development. Scientific Reports, 12.
[41] Gao Y, Li Y, Niu X, Wu Y, Guan X, Hong Y, et al. (2020). Identification and Validation of Prognostically Relevant Gene Signature in Melanoma. BioMed Research International, 2020(1), 5323614. https://doi.org/10.1155/2020/5323614
[42] Chen X, Liang S, Hao J, Wang T, HB, &Liu G, et al. Schlafen family is a prognostic biomarker and corresponds with immune infiltration in gastric cancer. Frontiers in immunology 13, 922138, doi:10.3389/fimmu.2022.922138 (2022).
[43] Hawkes JE, Cassidy PB, Manga P, Boissy RE, Goldgar D, Cannon-Albright L, et al. (2013). Report of a novel OCA2 gene mutation and an investigation of OCA2 variants on melanoma risk in a familial melanoma pedigree. Journal of Dermatological Science, 69(1), 30-37. https://doi.org/10.1016/j.jdermsci.2012.09.016
[44] Chahal HS, Lin Y, Ransohoff KJ, Hinds DA, Wu W, Dai H-J, et al. (2016). Genome-wide association study identifies novel susceptibility loci for cutaneous squamous cell carcinoma. Nature Communications, 7(1), 12048. https://doi.org/10.1038/ncomms12048
[45] Ma W, Jin H, Liu W, Li X, Zhou X, Guo X, et al. (2020). Homeobox B8 Targets Sterile Alpha Motif Domain-Containing Protein 9 and Drives Glioma Progression. Neuroscience Bulletin, 36(4), 359-371. https://doi.org/10.1007/s12264-019-00436-y
[46] Ma W, Zhang K, Bao Z, Jiang T, & Zhang Y. (2021). SAMD9 Is Relating With M2 Macrophage and Remarkable Malignancy Characters in Low-Grade Glioma [Original Research]. Frontiers in Immunology, Volume 12 - 2021. https://doi.org/10.3389/fimmu.2021.659659
[47] Gyrd-Hansen M, & Meier P. (2010). Erratum: IAPs: From caspase inhibitors to modulators of NF-κB, inflammation and cancer (Nature Reviews Cancer (2010) 10 (561-574)). Nature Reviews Cancer, 10(12), 890-890.
[48] Choi J, Hwang YK, Choi YJ, Yoo KE, Kim JH, Nam SJ, et al. (2007). Neuronal apoptosis inhibitory protein is overexpressed in patients with unfavorable prognostic factors in breast cancer. J Korean Med Sci, 22 Suppl(Suppl), S17-23. https://doi.org/10.3346/jkms.2007.22.S.S17
[49] Yang L, Zhao W, Wei P, Zuo W & Zhu S. Tumor suppressor p53 induces miR-15a processing to inhibit neuronal apoptosis inhibitory protein (NAIP) in the apoptotic response DNA damage in breast cancer cell. American journal of translational research 9, 683-691 (2017).
[50] Xiao Y, & Yu D. (2021). Tumor microenvironment as a therapeutic target in cancer. Pharmacology & Therapeutics, 221, 107753. https://doi.org/https://doi.org/10.1016/j.pharmthera.2020.107753
[51] de Visser KE, & Joyce JA. (2023). The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell, 41(3), 374-403. https://doi.org/10.1016/j.ccell.2023.02.016
[52] Marzagalli M, Ebelt ND, & Manuel ER. (2019). Unraveling the crosstalk between melanoma and immune cells in the tumor microenvironment. Seminars in Cancer Biology, 59, 236-250. https://doi.org/https://doi.org/10.1016/j.semcancer.2019.08.002
[53] Cui J, Chen Y, Wang HY, & Wang R-F. (2014). Mechanisms and pathways of innate immune activation and regulation in health and cancer. Human Vaccines & Immunotherapeutics, 10(11), 3270-3285. https://doi.org/10.4161/21645515.2014.979640
[54] Woo S-R, Fuertes Mercedes B, Corrales L, Spranger S, Furdyna Michael J, Leung Michael YK, et al. (2015). STING-Dependent Cytosolic DNA Sensing Mediates Innate Immune Recognition of Immunogenic Tumors. Immunity, 42(1), 199. https://doi.org/10.1016/j.immuni.2014.12.015
Type
Published
Data Availability Statement
The data that support the findings of this study are available in the following repositories:
TCGA: http://cancergenome.nih.gov
GEO: https://www.ncbi.nlm.nih.gov/geo
Issue
Section
License
Copyright (c) 2025 Life Conflux

This work is licensed under a Creative Commons Attribution 4.0 International License.







