New Insights into Influenza Virus-Host Interactions, ImmuneResponses, and Vaccine Development at Single-Cell Resolution
DOI:
https://doi.org/10.71321/dz9d7b09Keywords:
Single-cell sequencing technology, Influenza virus, Cellular heterogeneity, Immune cell subset differentiation, Influenza vaccineAbstract
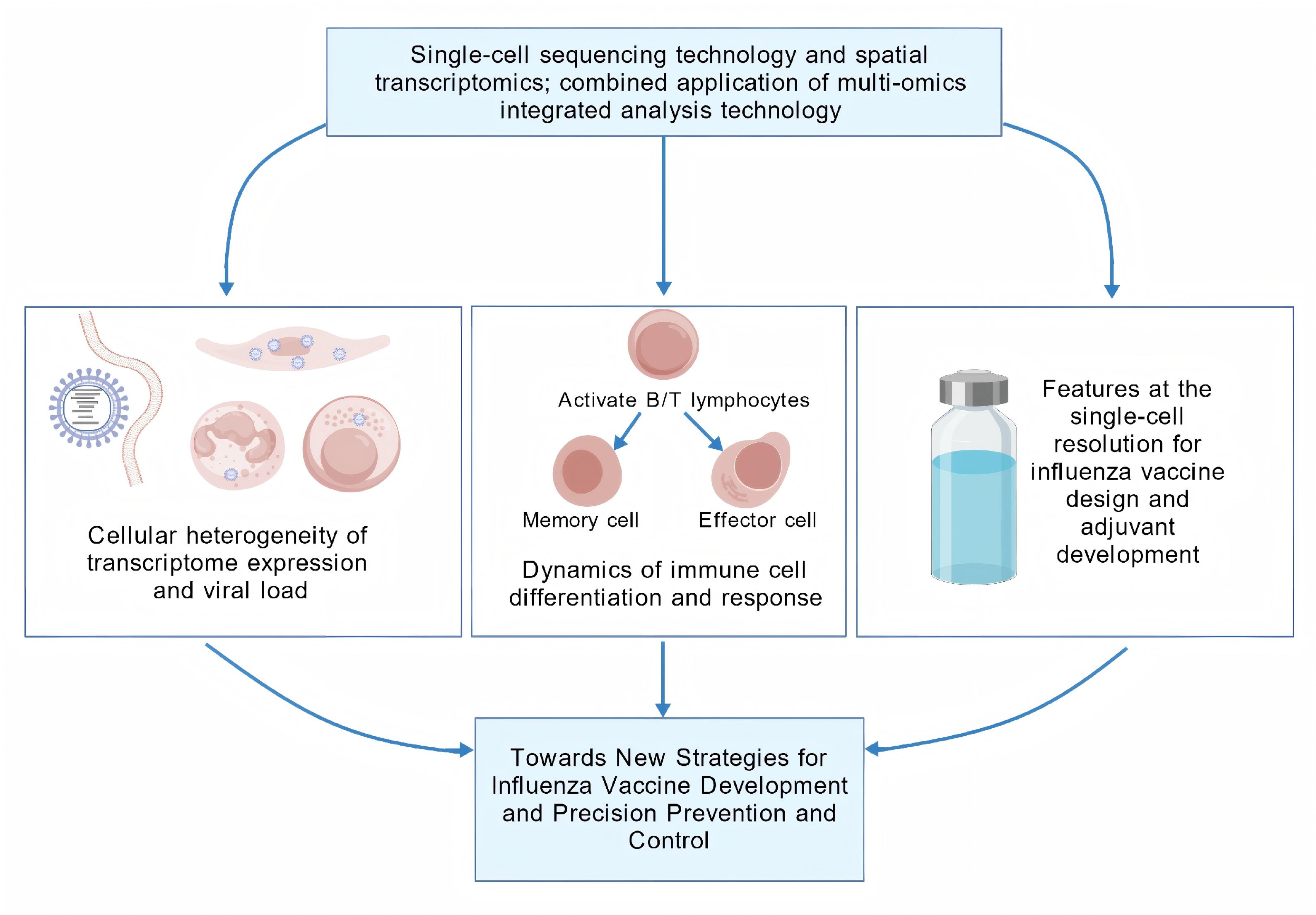
The integration of single-cell sequencing with spatial transcriptomics and multi-omics analyses has enabled a paradigm shift in biomedical research, thereby expanding its applicability and scientific impact. In the context of influenza virus studies, this technology has been instrumental in dissecting cellular heterogeneity, as demonstrated by its capacity to analyze differential transcriptomic profiles and reconstruct differentiation trajectories at the single-cell level following viral infection. These advances have provided mechanistic insights and a holistic understanding of influenza pathogenesis, surpassing the limitations of bulk-level analyses. This review provides a comprehensive dissection of cutting-edge applications of single-cell sequencing in elucidating influenza virus infection mechanisms, immune cell heterogeneity, and vaccine development. By highlighting the single-cell resolution of virus–host interactions and vaccine efficacy studies, this work offers novel perspectives for designing precision-targeted antiviral interventions.
References
[1] Catalina Pardo-Roa, Martha I Nelson, Naomi Ariyama, Carolina Aguayo, Leonardo I Almonacid, Ana S Gonzalez-Reiche, et al. Cross-species and mammal-to-mammal transmission of clade 2.3.4.4b highly pathogenic avian influenza A/H5N1 with PB2 adaptations. Nat Commun. 2025;16(1):2232.https://doi.org/10.1038/s41467-025-57338-z
[2] Hyunsuh Kim, Robert G Webster, Richard J Webby. Influenza Virus: Dealing with a Drifting and Shifting Pathogen. Viral Immunol. 2018;31(2):174-183. https://doi.org/10.1089/vim.2017.0141
[3] Tomoko Sumitomo, Shigetada Kawabata. Respiratory tract barrier dysfunction in viral-bacterial co-infection cases. Jpn Dent Sci Rev. 2024;60:44-52. https://doi.org/10.1016/j.jdsr.2023.12.006
[4] Anshu P Gounder, Adrianus C M Boon. Influenza Pathogenesis: The Effect of Host Factors on Severity of Disease. J Immunol. 2019;202(2):341-350.https://doi.org/10.4049/jimmunol.1801010
[5] Matloob Husain. Host factors involved in influenza virus infection. Emerg Top Life Sci. 2020;4(4):389-398. https://doi.org/10.1042/ETLS20200232
[6] Praveen M Varghese, Uday Kishore, Reena Rajkumari. Innate and adaptive immune responses against Influenza A Virus: Immune evasion and vaccination strategies. Immunobiology. 2022;227(6):152279. https://doi.org/10.1016/j.imbio.2022.152279
[7] Thi H O Nguyen, Louise C Rowntree, Brendon Y Chua, Ryan S Thwaites, Katherine Kedzierska. Defining the balance between optimal immunity and immunopathology in influenza virus infection. Nat Rev Immunol. 2024;24(10):720-735. https://doi.org/10.1038/s41577-024-01029-1
[8] Marta De Angelis, Paola Checconi, David Olagnier. Editorial: Host-cell pathways modulated by influenza virus infection: new insight into pathogenetic mechanisms and cell-targeted antiviral strategies. Front Cell Infect Microbiol. 2024;14:1372896. https://doi.org/10.3389/fcimb.2024.1372896
[9] Lu Zhang, Yueying Yang, Jianjun Tan. Applications and emerging challenges of single-cell RNA sequencing technology in tumor drug discovery. Drug Discov Today. 2025;30(2):104290. https://doi.org/10.1016/j.drudis.2025.104290
[10] Koen Van den Berge, Fanny Perraudeau, Charlotte Soneson, Michael I Love, Davide Risso, Jean-Philippe Vert, et al. Observation weights unlock bulk RNA-seq tools for zero inflation and single-cell applications. Genome Biol. 2018;19(1):24. https://doi.org/10.1186/s13059-018-1406-4
[11] Qing'e Shan, Jiahuang Qiu, Zheng Dong, Xiaotong Xu, Shuping Zhang, Juan Ma, et al. Lung Immune Cell Niches and the Discovery of New Cell Subtypes. Adv Sci (Weinh). 2024;11(45):e2405490. https://doi.org/10.1002/advs.202405490
[12] Jeongwoo Lee, Do Young Hyeon, Daehee Hwang. Single-cell multiomics: technologies and data analysis methods. Exp Mol Med. 2020;52(9):1428-1442. https://doi.org/10.1038/s12276-020-0420-2
[13] Xiangyu Wu, Xin Yang, Yunhan Dai, Zihan Zhao, Junmeng Zhu, Hongqian Guo, et al. Single-cell sequencing to multi-omics: technologies and applications. Biomark Res. 2024;12(1):110. https://doi.org/10.1186/s40364-024-00643-4
[14] Woosung Chung, Hye Hyeon Eum, Hae-Ock Lee, Kyung-Min Lee, Han-Byoel Lee, Kyu-Tae Kim, et al. Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nat Commun. 2017;8:15081. https://doi.org/10.1038/ncomms15081
[15] Jingchun Ma, Wei Jin, Li Rong, Zhanyu Gao, Zaman Hazrat, Hosen Md Shakhawat, et al. IT-scC&T-seq streamlines scalable, parallel profiling of protein-DNA interactions in single cells. Genome Biol. 2025;26(1):196. https://doi.org/10.1186/s13059-025-03661-z
[16] Elisabet Rosàs-Canyelles, Andrew J Modzelewski, Alisha Geldert, Lin He, Amy E Herr. Assessing heterogeneity among single embryos and single blastomeres using open microfluidic design. Sci Adv. 2020;6(17):eaay1751.https://doi.org/10.1126/sciadv.aay1751
[17] Diether Lambrechts, Els Wauters, Bram Boeckx, Sara Aibar, David Nittner, Oliver Burton, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med. 2018;24(8):1277-1289. https://doi.org/10.1038/s41591-018-0096-5
[18] Fengying Sun, Haoyan Li, Dongqing Sun, Shaliu Fu, Lei Gu, Xin Shao, et al. Single-cell omics: experimental workflow, data analyses and applications. Sci China Life Sci. 2025;68(1):5-102. https://doi.org/10.1007/s11427-023-2561-0
[19] Malte D Luecken, Fabian J Theis. Current best practices in single-cell RNA-seq analysis: a tutorial. Mol Syst Biol. 2019;15(6):e8746.https://doi.org/10.15252/msb.20188746
[20] Andre Gross, Jonas Schoendube, Stefan Zimmermann, Maximilian Steeb, Roland Zengerle, Peter Koltay. Technologies for Single-Cell Isolation. Int J Mol Sci. 2015;16(8):16897-16919. https://doi.org/10.3390/ijms160816897
[21] Val Yianni, Paul T Sharpe. Single Cell RNA-Seq: Cell Isolation and Data Analysis. Methods Mol Biol. 2022;2403:81-89. https://doi.org/10.1007/978-1-0716-1847-9_7
[22] Wenbo Guo, Yining Hu, Jingyang Qian, Lidan Zhu, Junyun Cheng, Jie Liao, et al. Laser capture microdissection for biomedical research: towards high-throughput, multi-omics, and single-cell resolution. J Genet Genomics. 2023;50(9):641-651.https://doi.org/10.1016/j.jgg.2023.07.011
[23] Katherine M McKinnon. Flow Cytometry: An Overview. Curr Protoc Immunol. 2018;120:5.1.1-5.1.11. https://doi.org/10.1002/cpim.40
[24] Joy A Pai, Ansuman T Satpathy. High-throughput and single-cell T cell receptor sequencing technologies. Nat Methods. 2021;18(8):881-892. https://doi.org/10.1038/s41592-021-01201-8
[25] Xuhao Luo, Jui-Yi Chen, Marzieh Ataei, Abraham Lee. Microfluidic Compartmentalization Platforms for Single Cell Analysis. Biosensors (Basel). 2022;12(2):58. https://doi.org/10.3390/bios12020058
[26] Jiayi Peng, Feng Li, Xiangdong Xu, Shen Hu. Single-Cell Analysis of circRNA Using ddPCR. Methods Mol Biol. 2023;2689:169-177. https://doi.org/10.1007/978-1-0716-3323-6_13
[27] Evan Z Macosko, Anindita Basu, Rahul Satija, James Nemesh, Karthik Shekhar, Melissa Goldman, et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell. 2015;161(5):1202-1214. https://doi.org/10.1016/j.cell.2015.05.002
[28] Grace X Y Zheng, Jessica M Terry, Phillip Belgrader, Paul Ryvkin, Zachary W Bent, Ryan Wilson, et al. Massively parallel digital transcriptional profiling of single cells. Nat Commun. 2017;8:14049. https://doi.org/10.1038/ncomms14049
[29] Yiming Wang, Yousu Wang, Xiaojie Wang, Wei Sun, Fengrui Yang, Xuebiao Yao, et al. Label-free active single-cell encapsulation enabled by microvalve-based on-demand droplet generation and real-time image processing. Talanta. 2024;276:126299. https://doi.org/10.1016/j.talanta.2024.126299
[30] Todd M Gierahn, Marc H Wadsworth, Travis K Hughes, Bryan D Bryson, Andrew Butler, Rahul Satija, et al. Seq-Well: portable, low-cost RNA sequencing of single cells at high throughput. Nat Methods. 2017;14(4):395-398. https://doi.org/10.1038/nmeth.4179
[31] Aleksandra A Kolodziejczyk, Jong Kyoung Kim, Valentine Svensson, John C Marioni, Sarah A Teichmann. The technology and biology of single-cell RNA sequencing. Mol Cell. 2015;58(4):610-620. https://doi.org/10.1016/j.molcel.2015.04.005
[32] Xiaoying Fan, Xiannian Zhang, Xinglong Wu, Hongshan Guo, Yuqiong Hu, Fuchou Tang, et al. Single-cell RNA-seq transcriptome analysis of linear and circular RNAs in mouse preimplantation embryos. Genome Biol. 2015;16(1):148. https://doi.org/10.1186/s13059-015-0706-1
[33] Haide Chen, Xiunan Fang, Jikai Shao, Qi Zhang, Liwei Xu, Jiaye Chen, et al. Pan-Cancer Single-Nucleus Total RNA Sequencing Using snHH-Seq. Adv Sci (Weinh). 2024;11(5):e2304755.https://doi.org/10.1002/advs.202304755
[34] Ziye Xu, Tianyu Zhang, Hongyu Chen, Yuyi Zhu, Yuexiao Lv, Shunji Zhang, et al. High-throughput single nucleus total RNA sequencing of formalin-fixed paraffin-embedded tissues by snRandom-seq. Nat Commun. 2023;14(1):2734.https://doi.org/10.1038/s41467-023-38409-5
[35] Kuanwei Sheng, Wenjian Cao, Yichi Niu, Qing Deng, Chenghang Zong. Effective detection of variation in single-cell transcriptomes using MATQ-seq. Nat Methods. 2017;14(3):267-270. https://doi.org/10.1038/nmeth.4145
[36] Diego Adhemar Jaitin, Ephraim Kenigsberg, Hadas Keren-Shaul, Naama Elefant, Franziska Paul, Irina Zaretsky, et al. Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types. Science. 2014;343(6172):776-779.https://doi.org/10.1126/science.1247651
[37] Jiayun Chen, Xingsong Li, Hongbin Zhong, Yuhuan Meng, Hongli Du. Systematic comparison of germline variant calling pipelines cross multiple next-generation sequencers. Sci Rep. 2019;9(1):9345. https://doi.org/10.1038/s41598-019-45835-3
[38] Jonathan Foox, Scott W Tighe, Charles M Nicolet, Justin M Zook, Marta Byrska-Bishop, Wayne E Clarke, et al. Author Correction: Performance assessment of DNA sequencing platforms in the ABRF Next-Generation Sequencing Study. Nat Biotechnol. 2021;39(11):1466. https://doi.org/10.1038/s41587-021-01122-z
[39] Nicholas J Loman, Raju V Misra, Timothy J Dallman, Chrystala Constantinidou, Saheer E Gharbia, John Wain, et al. Performance comparison of benchtop high-throughput sequencing platforms [published correction appears in Nat Biotechnol. 2012 Jun;30(6):562]. Nat Biotechnol. 2012;30(5):434-439. https://doi.org/10.1038/nbt.2198
[40] Shuangyu Han, Zhan Zhao, Lei Yang, Jie Huang, Yubao Wang, Jing Feng. The performance of metagenomic next-generation sequencing in diagnosing pulmonary infectious diseases using authentic clinical specimens: The Illumina platform versus the Beijing Genomics Institute platform. Front Pharmacol. 2023;14:1164633.https://doi.org/10.3389/fphar.2023.1164633
[41] Victoria Meslier, Benoit Quinquis, Kévin Da Silva, Florian Plaza Oñate, Nicolas Pons, Hugo Roume, et al. Benchmarking second and third-generation sequencing platforms for microbial metagenomics. Sci Data. 2022;9(1):694.https://doi.org/10.1038/s41597-022-01762-z
[42] Mian Umair Ahsan, Anagha Gouru, Joe Chan, Wanding Zhou, Kai Wang. A signal processing and deep learning framework for methylation detection using Oxford Nanopore sequencing. Nat Commun. 2024;15(1):1448. https://doi.org/10.1038/s41467-024-45778-y
[43] Guilherme de Sena Brandine, Andrew D Smith. Falco: high-speed FastQC emulation for quality control of sequencing data. F1000Res. 2019;8:1874.https://doi.org/10.12688/f1000research.21142.2
[44] Anthony M Bolger, Marc Lohse, Bjoern Usadel. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114-2120. https://doi.org/10.1093/bioinformatics/btu170
[45] Matthew D Young,Sam Behjati. SoupX removes ambient RNA contamination from droplet-based single-cell RNA sequencing data. Gigascience. 2020;9(12):giaa151. https://doi.org/10.1093/gigascience/giaa151
[46] Christoph Hafemeister, Rahul Satija. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 2019;20(1):296.https://doi.org/10.1186/s13059-019-1874-1
[47] Qingyang Yin, Yang Wang, Jinting Guan, Guoli Ji. scIAE: an integrative autoencoder-based ensemble classification framework for single-cell RNA-seq data. Brief Bioinform. 2022;23(1):bbab508. https://doi.org/10.1093/bib/bbab508
[48] F Alexander Wolf, Philipp Angerer, Fabian J Theis. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 2018;19(1):15.https://doi.org/10.1186/s13059-017-1382-0
[49] Alistair B Russell, Cole Trapnell, Jesse D Bloom. Extreme heterogeneity of influenza virus infection in single cells. Elife. 2018;7:e32303. https://doi.org/10.7554/eLife.32303
[50] Junsong Zhang, Jun Liu, Yaochang Yuan, Feng Huang, Rong Ma, Baohong Luo, et al. Two waves of pro-inflammatory factors are released during the influenza A virus (IAV)-driven pulmonary immunopathogenesis. PLoS Pathog. 2020;16(2):e1008334.https://doi.org/10.1371/journal.ppat.1008334
[51] Yael Steuerman, Merav Cohen, Naama Peshes-Yaloz, Liran Valadarsky, Ofir Cohn, Eyal David, et al. Dissection of Influenza Infection In Vivo by Single-Cell RNA Sequencing. Cell Syst. 2018;6(6):679-691.e4. https://doi.org/10.1016/j.cels.2018.05.008
[52] Susanne Kessler, Bradly Burke, Geoffroy Andrieux, Jan Schinköthe, Lea Hamberger, Johannes Kacza, et al. Deciphering bat influenza H18N11 infection dynamics in male Jamaican fruit bats on a single-cell level. Nat Commun. 2024;15(1):4500. https://doi.org/10.1038/s41467-024-48934-6
[53] Jiamin Gao, Jing Wei, Simei Qin, Sheng Liu, Shuangyan Mo, Qian Long, et al. Exploring the global immune landscape of peripheral blood mononuclear cells in H5N6-infected patient with single-cell transcriptomics. BMC Med Genomics. 2023;16(1):249. https://doi.org/10.1186/s12920-023-01693-7
[54] Qiwen Shi, Pengfei Zhang, Qingtao Hu, Tianxin Zhang, Ruixia Hou, Shengxiang Yin, et al. Role of TOMM34 on NF-κB activation-related hyperinflammation in severely ill patients with COVID-19 and influenza. EBioMedicine. 2024;108:105343. https://doi.org/10.1016/j.ebiom.2024.105343
[55] Shaoyan Gu, Wenxuan Xiao, Zhongkuo Yu, Jia Xiao, Mingze Sun, Lu Zhang, et al. Single-cell RNA-seq reveals the immune response of Co-infection with streptococcus pneumoniae after influenza A virus by a lung-on-chip: The molecular structure and mechanism of tight junction protein ZO-1. Int J Biol Macromol. 2025;306(Pt 4):141815. https://doi.org/10.1016/j.ijbiomac.2025.141815
[56] Linnan Zhu, Penghui Yang, Yingze Zhao, Zhenkun Zhuang, Zhifeng Wang, Rui Song, et al. Single-Cell Sequencing of Peripheral Mononuclear Cells Reveals Distinct Immune Response Landscapes of COVID-19 and Influenza Patients. Immunity. 2020;53(3):685-696.e3. https://doi.org/10.1016/j.immuni.2020.07.009
[57] Yixin Zou, Xifang Sun, Yifan Wang, Yidi Wang, Xiangyu Ye, Junlan Tu, et al. Integrating single-cell RNA sequencing data to genome-wide association analysis data identifies significant cell types in influenza A virus infection and COVID-19. Brief Funct Genomics. 2024;23(2):110-117. https://doi.org/10.1093/bfgp/elad025
[58] Jiapei Yu, Congcong Shang, Xiaoyan Deng, Ju Jia, Xiao Shang, Zeyi Wang, et al. Time-resolved scRNA-seq reveals transcription dynamics of polarized macrophages with influenza A virus infection and antigen presentation to T cells. Emerg Microbes Infect. 2024;13(1):2387450. https://doi.org/10.1080/22221751.2024.2387450
[59] Yin Zhang, Lu Zong, Yuanling Zheng, Yu Zhang, Nan Li, Yaoyao Li, et al. A single-cell atlas of the peripheral immune response in patients with influenza A virus infection. iScience. 2023;26(12):108507. https://doi.org/10.1016/j.isci.2023.108507
[60] Andrew Muir, Basudev Paudyal, Selma Schmidt, Ehsan Sedaghat-Rostami, Soumendu Chakravarti, Sonia Villanueva-Hernández, et al. Single-cell analysis reveals lasting immunological consequences of influenza infection and respiratory immunization in the pig lung. PLoS Pathog. 2024;20(7):e1011910. https://doi.org/10.1371/journal.ppat.1011910
[61] Jian Ge, Hongxia Shao, Hongxu Ding, Yuefeng Huang, Xuebing Wu, Jie Sun, et al. Single Cell Analysis of Lung Lymphatic Endothelial Cells and Lymphatic Responses during Influenza Infection. J Respir Biol Transl Med. 2024;1(1):10003.https://doi.org/10.35534/jrbtm.2024.10003
[62] Eriko Kudo, Eric Song, Laura J Yockey, Tasfia Rakib, Patrick W Wong, Robert J Homer, et al. Low ambient humidity impairs barrier function and innate resistance against influenza infection. Proc Natl Acad Sci U S A. 2019;116(22):10905-10910.https://doi.org/10.1073/pnas.1902840116
[63] Ju Jia, Hui Li, Zhisheng Huang, Jiapei Yu, Ying Zheng, Bin Cao. Comprehensive immune landscape of lung-resident memory CD8+ T cells after influenza infection and reinfection in a mouse model. Front Microbiol. 2023;14:1184884.https://doi.org/10.3389/fmicb.2023.1184884
[64] Mustafa H Ghanem, Andrew J Shih, Houman Khalili, Emily G Werth, Jayanta K Chakrabarty, Lewis M Brown, et al. Proteomic and Single-Cell Transcriptomic Dissection of Human Plasmacytoid Dendritic Cell Response to Influenza Virus. Front Immunol. 2022;13:814627.https://doi.org/10.3389/fimmu.2022.814627
[65] Jenny E Hernandez-Davies, Emmanuel P Dollinger, Egest J Pone, Jiin Felgner, Li Liang, Shirin Strohmeier, et al. Magnitude and breadth of antibody cross-reactivity induced by recombinant influenza hemagglutinin trimer vaccine is enhanced by combination adjuvants. Sci Rep. 2022;12(1):9198. https://doi.org/10.1038/s41598-022-12727-y
[66] Felix Horns, Cornelia L Dekker, Stephen R Quake. Memory B Cell Activation, Broad Anti-influenza Antibodies, and Bystander Activation Revealed by Single-Cell Transcriptomics. Cell Rep. 2020;30(3):905-913.e6. https://doi.org/10.1016/j.celrep.2019.12.063
[67] Moujtaba Y Kasmani, Paytsar Topchyan, Ashley K Brown, Ryan J Brown, Xiaopeng Wu, Yao Chen, et al. A spatial sequencing atlas of age-induced changes in the lung during influenza infection. Nat Commun. 2023;14(1):6597.https://doi.org/10.1038/s41467-023-42021-y
[68] Nimitha R Mathew,Jayalal K Jayanthan,Ilya V Smirnov,Jonathan L Robinson,Hannes Axelsson,Sravya S Nakka, et al. Single-cell BCR and transcriptome analysis after influenza infection reveals spatiotemporal dynamics of antigen-specific B cells. Cell Rep. 2022;41(9):111764. https://doi.org/10.1016/j.celrep.2022.111764
[69] Shazeb Ahmad, Jianhui Li, Joél Schaust, Anne-Sophie Gribling-Burrer, Nina Geiger, Sabine C Fischer, et al. Visualizing the transcription and replication of influenza A viral RNAs in cells by multiple direct RNA padlock probing and in situ sequencing (mudRapp-seq). Nucleic Acids Res. 2025;53(11):gkaf461. https://doi.org/10.1093/nar/gkaf461
[70] Florian Wimmers, Michele Donato, Alex Kuo, Tal Ashuach, Shakti Gupta, Chunfeng Li, et al. The single-cell epigenomic and transcriptional landscape of immunity to influenza vaccination. Cell. 2021;184(15):3915-3935.e21. https://doi.org/10.1016/j.cell.2021.05.039
[71] Matthew Esparza, Prasanna Bhat, Beatriz Ma Fontoura. Viral-host interactions during splicing and nuclear export of influenza virus mRNAs. Curr Opin Virol. 2022;55:101254. https://doi.org/10.1016/j.coviro.2022.101254
[72] Milagros Sempere Borau, Victor G Gisbert, Josephine von Kempis, Laura M Arroyo-Fernández, Samira Schiefer, David Alsteens, et al. Proximity labelling of internalizing influenza A viruses reveals a role for neogenin in virus uptake. PLoS Pathog. Published online July 7, 2025. https://doi.org/10.1371/journal.ppat.1013338
[73] Jason S Long, Efstathios S Giotis, Olivier Moncorgé, Rebecca Frise, Bhakti Mistry, Joe James, et al. Species difference in ANP32A underlies influenza A virus polymerase host restriction. Nature. 2016;529(7584):101-104. https://doi.org/10.1038/nature16474
[74] Wilhelm Bertrams, Katja Hönzke, Benedikt Obermayer, Mario Tönnies, Torsten T Bauer, Paul Schneider, et al. Transcriptomic comparison of primary human lung cells with lung tissue samples and the human A549 lung cell line highlights cell type specific responses during infections with influenza A virus. Sci Rep. 2022;12(1):20608. https://doi.org/10.1038/s41598-022-24792-4
[75] Alistair B Russell, Elizaveta Elshina, Jacob R Kowalsky, Aartjan J W Te Velthuis, Jesse D Bloom. Single-Cell Virus Sequencing of Influenza Infections That Trigger Innate Immunity. J Virol. 2019;93(14):e00500-19. https://doi.org/10.1128/JVI.00500-19
[76] Phan T, Fay EJ, Lee Z, Aron S, Hu WS, Langlois RA. Segment-specific kinetics of mRNA, cRNA and vRNA accumulation during influenza infection. J Virol. 2021;95(10):e02102-20. https://doi.org/10.1128/JVI.02102-20
[77] Nilsson-Payant BE, tenOever BR, Te Velthuis AJW. The Host Factor ANP32A Is Required for Influenza A Virus vRNA and cRNA Synthesis. J Virol. 2022;96(4):e0209221. https://doi.org/10.1128/jvi.02092-21
[78] Zhang L, Wang Y, Shao Y, Guo J, Gao GF, Deng T. Fine Regulation of Influenza Virus RNA Transcription and Replication by Stoichiometric Changes in Viral NS1 and NS2 Proteins. J Virol. 2023;97(5):e0033723. https://doi.org/10.1128/jvi.00337-23
[79] Lei Zhang, Qiuxian Yang, Yuekun Shao, Shenyang Ding, Jiamei Guo, George F Gao, et al. Influenza A virus NS2 protein acts on vRNA-resident polymerase to drive the transcription to replication switch. Nucleic Acids Res. 2025;53(3):gkaf027. https://doi.org/10.1093/nar/gkaf027
[80] Kewei Fan, Yinping Jia, Song Wang, Hua Li, Defeng Wu, Guoshun Wang, et al. Role of Itk signalling in the interaction between influenza A virus and T-cells. J Gen Virol. 2012;93(Pt 5):987-997. https://doi.org/10.1099/vir.0.041228-0
[81] Zixin Ni, Jinliang Wang, Xiaofei Yu, Yifan Wang, Jingfei Wang, Xijun He, et al. Influenza virus uses mGluR2 as an endocytic receptor to enter cells. Nat Microbiol. 2024;9(7):1764-1777.https://doi.org/10.1038/s41564-024-01713-x
[82] Upasana Kulkarni, Rachel L Zemans, Candice A Smith, Sherri C Wood, Jane C Deng, Daniel R Goldstein. Excessive neutrophil levels in the lung underlie the age-associated increase in influenza mortality. Mucosal Immunol. 2019;12(2):545-554.https://doi.org/10.1038/s41385-018-0115-3
[83] Meagan M Jenkins, Holly Bachus, Davide Botta, Michael D Schultz, Alexander F Rosenberg, Beatriz León, et al. Lung dendritic cells migrate to the spleen to prime long-lived TCF1hi memory CD8+ T cell precursors after influenza infection. Sci Immunol. 2021;6(63):eabg6895.https://doi.org/10.1126/sciimmunol.abg6895
[84] Michael J Hogan, Nikita Maheshwari, Bridget E Begg, Annalisa Nicastri, Emma J Hedgepeth, Hiromi Muramatsu, et al. Cryptic MHC-E epitope from influenza elicits a potent cytolytic T cell response. Nat Immunol. 2023;24(11):1933-1946. https://doi.org/10.1038/s41590-023-01644-5
[85] Frederick Masson, Adele M Mount, Nicholas S Wilson, Gabrielle T Belz. Dendritic cells: driving the differentiation programme of T cells in viral infections. Immunol Cell Biol. 2008;86(4):333-342. https://doi.org/10.1038/icb.2008.15
[86] Julie Helft, Balaji Manicassamy, Pierre Guermonprez, Daigo Hashimoto, Aymeric Silvin, Judith Agudo, et al. Cross-presenting CD103+ dendritic cells are protected from influenza virus infection. J Clin Invest. 2012;122(11):4037-4047. https://doi.org/10.1172/JCI60659
[87] Samuel Amoah, Weiping Cao, Ekramy E Sayedahmed, Yuanyuan Wang, Amrita Kumar, Margarita Mishina, et al. The frequency and function of nucleoprotein-specific CD8+ T cells are critical for heterosubtypic immunity against influenza virus infection. J Virol. 2024;98(8):e0071124. https://doi.org/10.1128/jvi.00711-24
[88] Caroline M Finn,K Kai McKinstry. Ex Pluribus Unum: The CD4 T Cell Response against Influenza A Virus. Cells. 2024;13(7):639.https://doi.org/10.3390/cells13070639
[89] Christine Nguyen, Matthew Kudek, Ryan Zander, Hongshen Niu, Jian Shen, Ashley Bauer, et al. Bhlhe40 Promotes CD4+ T Helper 1 Cell and Suppresses T Follicular Helper Cell Differentiation during Viral Infection. J Immunol. 2024;212(11):1829-1842. https://doi.org/10.4049/jimmunol.2300355
[90] Paul R Dunbar, Emily K Cartwright, Alexander N Wein, Tetsuo Tsukamoto, Zheng-Rong Tiger Li, Nivedha Kumar, et al. Pulmonary monocytes interact with effector T cells in the lung tissue to drive TRM differentiation following viral infection. Mucosal Immunol. 2020;13(1):161-171. https://doi.org/10.1038/s41385-019-0224-7
[91] Yuki Muroyama, E John Wherry. Memory T-Cell Heterogeneity and Terminology. Cold Spring Harb Perspect Biol. 2021;13(10):a037929.https://doi.org/10.1101/cshperspect.a037929
[92] Josien Lanfermeijer, Koen van de Ven, Marion Hendriks, Harry van Dijken, Stefanie Lenz, Martijn Vos, et al. The Memory-CD8+-T-Cell Response to Conserved Influenza Virus Epitopes in Mice Is Not Influenced by Time Since Previous Infection. Vaccines (Basel). 2024;12(4):419.https://doi.org/10.3390/vaccines12040419
[93] Xin Chen, Mustafa Ghanizada, Vamsee Mallajosyula, Elsa Sola, Robson Capasso, Karan Raj Kathuria, et al. Differential roles of human CD4+ and CD8+ regulatory T cells in controlling self-reactive immune responses. Nat Immunol. 2025;26(2):230-239. https://doi.org/10.1038/s41590-024-02062-x
[94] Zhongfang Wang, Lingyan Zhu, Thi H O Nguyen, Yanmin Wan, Sneha Sant, Sergio M Quiñones-Parra, et al. Clonally diverse CD38+HLA-DR+CD8+ T cells persist during fatal H7N9 disease. Nat Commun. 2018;9(1):824.https://doi.org/10.1038/s41467-018-03243-7
[95] Li Shen, Jing Yang, Jin Liu, Yue Zhang, Yue Wu, Hong Xu. Thioredoxin regulates T cell proliferation and aggravates the severity of influenza a virus infection. Sci Rep. 2025;15(1):24419.https://doi.org/10.1038/s41598-025-10676-w
[96] Nicole M Arroyo-Díaz, Holly Bachus, Amber Papillion, Troy D Randall, Jobaida Akther, Alexander F Rosenberg, et al. Interferon-γ production by Tfh cells is required for CXCR3+ pre-memory B cell differentiation and subsequent lung-resident memory B cell responses. Immunity. 2023;56(10):2358-2372.e5. https://doi.org/10.1016/j.immuni.2023.08.015
[97] Kaitlin A Read, Stephanie A Amici, Sadaf Farsi, Madeline Cutcliffe, Bella Lee, Chan-Wang Jerry Lio, et al. PRMT5 Promotes T follicular helper Cell Differentiation and Germinal Center Responses during Influenza Virus Infection. J Immunol. 2024;212(9):1442-1449. https://doi.org/10.4049/jimmunol.2300270
[98] Adrien Sprumont, Ana Rodrigues, Simon J McGowan, Colin Bannard, Oliver Bannard. Germinal centers output clonally diverse plasma cell populations expressing high- and low-affinity antibodies. Cell. 2023;186(25):5486-5499.e13. https://doi.org/10.1016/j.cell.2023.10.022
[99] Miles B Horton, HoChan Cheon, Ken R Duffy, Daniel Brown, Shalin H Naik, Carolina Alvarado, et al. Lineage tracing reveals B cell antibody class switching is stochastic, cell-autonomous, and tuneable. Immunity. 2022;55(10):1843-1855.e6. https://doi.org/10.1016/j.immuni.2022.08.004
[100] Ji Eun Oh, Eric Song, Miyu Moriyama, Patrick Wong, Sophia Zhang, Ruoyi Jiang, et al. Intranasal priming induces local lung-resident B cell populations that secrete protective mucosal antiviral IgA. Sci Immunol. 2021;6(66):eabj5129. https://doi.org/10.1126/sciimmunol.abj5129
[101] Kosuke Miyauchi, Yu Adachi, Keisuke Tonouchi, Taiki Yajima, Yasuyo Harada, Hidehiro Fukuyama, et al. Influenza virus infection expands the breadth of antibody responses through IL-4 signalling in B cells. Nat Commun. 2021;12(1):3789. https://doi.org/10.1038/s41467-021-24090-z
[102] Namita T Gupta, Jason A Vander Heiden, Mohamed Uduman, Daniel Gadala-Maria, Gur Yaari, Steven H Kleinstein. Change-O: a toolkit for analyzing large-scale B cell immunoglobulin repertoire sequencing data. Bioinformatics. 2015;31(20):3356-3358.https://doi.org/10.1093/bioinformatics/btv359
[103] Jason A Vander Heiden, Gur Yaari, Mohamed Uduman, Joel N H Stern, Kevin C O'Connor, David A Hafler, et al. pRESTO: a toolkit for processing high-throughput sequencing raw reads of lymphocyte receptor repertoires. Bioinformatics. 2014;30(13):1930-1932. https://doi.org/10.1093/bioinformatics/btu138
[104] Nicholas Borcherding,Nicholas L Bormann,Gloria Kraus. scRepertoire: An R-based toolkit for single-cell immune receptor analysis. F1000Res. 2020;9:47.https://doi.org/10.12688/f1000research.22139.2
[105] Kelly Street, Davide Risso, Russell B Fletcher, Diya Das, John Ngai, Nir Yosef, et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics. 2018;19(1):477. https://doi.org/10.1186/s12864-018-4772-0
[106] Volker Bergen, Marius Lange, Stefan Peidli, F Alexander Wolf, Fabian J Theis. Generalizing RNA velocity to transient cell states through dynamical modeling. Nat Biotechnol. 2020;38(12):1408-1414. https://doi.org/10.1038/s41587-020-0591-3
[107] Yuexin Wang, Qiuli Yang, Yingjie Dong, Likun Wang, Zhiyuan Zhang, Ruiying Niu, et al. Piezo1-directed neutrophil extracellular traps regulate macrophage differentiation during influenza virus infection. Cell Death Dis. 2025;16(1):60. https://doi.org/10.1038/s41419-025-07395-5
[108] Paul M Jordan, Kerstin Günther, Vivien Nischang, Yuping Ning, Stefanie Deinhardt-Emmer, Christina Ehrhardt, et al. Influenza A virus selectively elevates prostaglandin E2 formation in pro-resolving macrophages. iScience. 2023;27(1):108775. https://doi.org/10.1016/j.isci.2023.108775
[109] Erin Crossey, Senegal Carty, Fengzhi Shao, Jhonatan Henao-Vasquez, Alexandra B Ysasi, Michelle Zeng, et al. Influenza induces lung lymphangiogenesis independent of YAP/TAZ activity in lymphatic endothelial cells. Sci Rep. 2024;14(1):21324. Published 2024 Sep 12. https://doi.org/10.1038/s41598-024-72115-6
[110] Melissa Swiecki, Marco Colonna. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. 2015;15(8):471-485. https://doi.org/10.1038/nri3865
[111] Duojiao Wu, David E Sanin, Bart Everts, Qiongyu Chen, Jing Qiu, Michael D Buck, et al. Type 1 Interferons Induce Changes in Core Metabolism that Are Critical for Immune Function. Immunity. 2016;44(6):1325-1336. https://doi.org/10.1016/j.immuni.2016.06.006
[112] Anna Aiello, Anna Calabrò, Mattia Emanuela Ligotti, Giulia Accardi, Mojtaba Shekarkar Azgomi, Nadia Caccamo, et al. Enhancing flu vaccine responses in older adults: preliminary insights from the ISOLDA study on immunosenescence and antioxidant and anti-inflammatory approaches. Immun Ageing. 2025;22(1):13. Published 2025 Mar 26. https://doi.org/10.1186/s12979-025-00506-y
[113] Nicholas S Rhoades, Michael Davies, Sloan A Lewis, Isaac R Cinco, Steven G Kohama, Luiz E Bermudez, et al. Functional, transcriptional, and microbial shifts associated with healthy pulmonary aging in rhesus macaques. Cell Rep. 2022;39(3):110725. https://doi.org/10.1016/j.celrep.2022.110725
[114] Yufei Wang, Ronghong Li, Renyang Tong, Taiwei Chen, Mingze Sun, Lingjie Luo, et al. Integrating single-cell RNA and T cell/B cell receptor sequencing with mass cytometry reveals dynamic trajectories of human peripheral immune cells from birth to old age. Nat Immunol. 2025;26(2):308-322. https://doi.org/10.1038/s41590-024-02059-6
[115] Burton AR, Guillaume SM, Foster WS,Adam K Wheatley,Danika L Hill,Edward J Carr, et al. The memory B cell response to influenza vaccination is impaired in older persons. Cell Rep. 2024;43(2):113745.https://doi.org/10.1016/j.celrep.2024.113745
[116] Meng Wang, Ruoyi Jiang, Subhasis Mohanty, Hailong Meng, Albert C Shaw, Steven H Kleinstein. High-throughput single-cell profiling of B cell responses following inactivated influenza vaccination in young and older adults. Aging (Albany NY). 2023;15(18):9250-9274.https://doi.org/10.18632/aging.204778
[117] Adinda Kok, Rachel Scheuer, Theo M Bestebroer, David F Burke, Samuel H Wilks, Monique I Spronken, et al. Characterization of A/H7 influenza virus global antigenic diversity and key determinants in the hemagglutinin globular head mediating A/H7N9 antigenic evolution. mBio. 2023;14(5):e0048823. https://doi.org/10.1128/mbio.00488-23
[118] Caryn Myn Li Lim, Thamil Vaani Komarasamy, Nur Amelia Azreen Binti Adnan, Ammu Kutty Radhakrishnan, Vinod R M T Balasubramaniam. Recent Advances, Approaches and Challenges in the Development of Universal Influenza Vaccines. Influenza Other Respir Viruses. 2024;18(3):e13276. https://doi.org/10.1111/irv.13276
[119] HIPC-CHI Signatures Project Team; HIPC-I Consortium. Multicohort analysis reveals baseline transcriptional predictors of influenza vaccination responses. Sci Immunol. 2017;2(14):eaal4656. https://doi.org/10.1126/sciimmunol.aal4656
[120] Lukas Hoen, Sarah Lartey, Fan Zhou, Rishi D Pathirana, Florian Krammer, Kristin G-I Mohn, et al. Impact of Pre-Existing Immunity and Age on Antibody Responses to Live Attenuated Influenza Vaccine. Vaccines (Basel). 2024;12(8):864. https://doi.org/10.3390/vaccines12080864
[121] Paul J Turner, Jo Southern, Nick J Andrews, Elizabeth Miller, Michel Erlewyn-Lajeunesse. Safety of live attenuated influenza vaccine in young people with egg allergy: multicentre prospective cohort study. BMJ. 2015;351:h6291.https://doi.org/10.1136/bmj.h6291
[122] Jenna J Guthmiller, Linda Yu-Ling Lan, Lei Li, Yanbin Fu, Sean A Nelson, Carole Henry, et al. Long-lasting B cell convergence to distinct broadly reactive epitopes following vaccination with chimeric influenza virus hemagglutinins. Immunity. 2025;58(4):980-996.e7. https://doi.org/10.1016/j.immuni.2025.02.025
[123] Yi Wang, Xiaoxia Wang, Xinbei Jia, Jieqiong Li, Jin Fu, Xiaolan Huang, et al. Influenza vaccination features revealed by a single-cell transcriptome atlas. J Med Virol. 2023;95(1):e28174.https://doi.org/10.1002/jmv.28174
[124] Anoma Nellore, Esther Zumaquero, Christopher D Scharer, Christopher F Fucile, Christopher M Tipton, R Glenn King, et al. A transcriptionally distinct subset of influenza-specific effector memory B cells predicts long-lived antibody responses to vaccination in humans. Immunity. 2023;56(4):847-863.e8. https://doi.org/10.1016/j.immuni.2023.03.001
[125] Grohskopf LA, Ferdinands JM, Blanton LH, Broder KR, Loehr J. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2024-25 Influenza Season. MMWR Recomm Rep. 2024;73(5):1-25. Published 2024 Aug 29. https://doi.org/10.15585/mmwr.rr7305a1
[126] Melissa K Andrew, Vivek Shinde, Lingyun Ye, Todd Hatchette, François Haguinet, Gael Dos Santos, et al. The Importance of Frailty in the Assessment of Influenza Vaccine Effectiveness Against Influenza-Related Hospitalization in Elderly People. J Infect Dis. 2017;216(4):405-414. https://doi.org/10.1093/infdis/jix282
[127] Lorry G Rubin, Myron J Levin, Per Ljungman, E Graham Davies, Robin Avery, Marcie Tomblyn, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3):309-318. https://doi.org/10.1093/cid/cit816
[128] Influenza in Pregnancy: Prevention and Treatment: ACOG Committee Statement No. 7. Obstet Gynecol. 2024;143(2):e24-e30. https://doi.org/10.1097/AOG.0000000000005479
[129] Clio Bilotta, Giulio Perrone, Valeria Adelfio, Giovanni Francesco Spatola, Maria Laura Uzzo, Antonina Argo, et al. COVID-19 Vaccine-Related Thrombosis: A Systematic Review and Exploratory Analysis. Front Immunol. 2021;12:729251. https://doi.org/10.3389/fimmu.2021.729251
[130] Anna Schmidt, Jana Fuchs, Mark Dedden, Katharina Kocher, Christine Schülein, Julian Hübner, et al. Inflammatory conditions shape phenotypic and functional characteristics of lung-resident memory T cells in mice. Nat Commun. 2025;16(1):3612. https://doi.org/10.1038/s41467-025-58931-y
[131] Brittany L Hartwell, Mariane B Melo, Peng Xiao, Ashley A Lemnios, Na Li, Jason Y H Chang, et al. Intranasal vaccination with lipid-conjugated immunogens promotes antigen transmucosal uptake to drive mucosal and systemic immunity. Sci Transl Med. 2022;14(654):eabn1413. https://doi.org/10.1126/scitranslmed.abn1413
[132] Samuel W Kazer, Colette Matysiak Match, Erica M Langan, Marie-Angèle Messou, Thomas J LaSalle, Elise O'Leary, et al. Primary nasal influenza infection rewires tissue-scale memory response dynamics. Immunity. 2024;57(8):1955-1974.e8. https://doi.org/10.1016/j.immuni.2024.06.005
[133] Lisa A Jackson, Wilbur H Chen, Jack T Stapleton, Cornelia L Dekker, Anna Wald, Rebecca C Brady, et al. Immunogenicity and safety of varying dosages of a monovalent 2009 H1N1 influenza vaccine given with and without AS03 adjuvant system in healthy adults and older persons. J Infect Dis. 2012;206(6):811-820. https://doi.org/10.1093/infdis/jis427
[134] Lei Feng, Na Han, Yu-Bo Han, Meng-Wen Shang, Teng-Wei Liang, Zhi-Hui Liu, et al. Structural analysis of a soluble polysaccharide GSPA-0.3 from the root of Panax ginseng C. A. Meyer and its adjuvant activity with mechanism investigation. Carbohydr Polym. 2024;326:121591. https://doi.org/10.1016/j.carbpol.2023.121591
[135] Trine Sundebo Meldgaard, Fabiola Blengio, Denise Maffione, Chiara Sammicheli, Simona Tavarini, Sandra Nuti, et al. Single-Cell Analysis of Antigen-Specific CD8+ T-Cell Transcripts Reveals Profiles Specific to mRNA or Adjuvanted Protein Vaccines. Front Immunol. 2021;12:757151. https://doi.org/10.3389/fimmu.2021.757151
[136] Menghua Lyu, Xuyang Shi, Yang Liu, Hongyan Zhao, Yue Yuan, Run Xie, et al. Single-Cell Transcriptome Analysis of H5N1-HA-Stimulated Alpaca PBMCs. Biomolecules. 2022;13(1):60. https://doi.org/10.3390/biom13010060
[137] Xiangyu Ye, Sheng Yang, Junlan Tu, Lei Xu, Yifan Wang, Hongbo Chen, et al. Leveraging baseline transcriptional features and information from single-cell data to power the prediction of influenza vaccine response. Front Cell Infect Microbiol. 2024;14:1243586. https://doi.org/10.3389/fcimb.2024.1243586
[138] Can Ergen, Valeh Valiollah Pour Amiri, Martin Kim, Ori Kronfeld, Aaron Streets, Adam Gayoso, et al. Scvi-hub: an actionable repository for model-driven single-cell analysis. Nat Methods. 2025;22(9):1836-1845. https://doi.org/10.1038/s41592-025-02799-9
[139] Lucy Her, Hao-Jie Zhu. Carboxylesterase 1 and Precision Pharmacotherapy: Pharmacogenetics and Nongenetic Regulators [published correction appears in Drug Metab Dispos. 2020 Nov;48(11):1246. doi: 10.1124/dmd.119.089680err.]. Drug Metab Dispos. 2020;48(3):230-244. https://doi.org/10.1124/dmd.119.089680
[140] Syed M Moin, Jeffrey C Boyington, Seyhan Boyoglu-Barnum, Rebecca A Gillespie, Gabriele Cerutti, Crystal Sao-Fong Cheun, et al. Co-immunization with hemagglutinin stem immunogens elicits cross-group neutralizing antibodies and broad protection against influenza A viruses. Immunity. 2022;55(12):2405-2418.e7. https://doi.org/10.1016/j.immuni.2022.10.015
[141] Dania Zhivaki, Stephanie N Kennedy, Josh Park, Francesco Boriello, Pascal Devant, Anh Cao, et al. Correction of age-associated defects in dendritic cells enables CD4+ T cells to eradicate tumors. Cell. 2024;187(15):3888-3903.e18. https://doi.org/10.1016/j.cell.2024.05.026
[142] Yaxu Liang, Xuejiao Zhu, Ruhao Zhuo, Ning Peng, Shuyu Chen, Shimeng Huang, et al. The role of m6A RNA methylation in a love-hate relationship between porcine rotavirus and host cells. Cell Biosci. 2025;15(1):99. https://doi.org/10.1186/s13578-025-01436-4
[143] Michelle M Li, Yepeng Huang, Marissa Sumathipala, Man Qing Liang, Alberto Valdeolivas, Ashwin N Ananthakrishnan, et al. Contextual AI models for single-cell protein biology. Nat Methods. 2024;21(8):1546-1557.https://doi.org/10.1101/2023.07.18.549602
[144] Tommy K Cheung, Chien-Yun Lee, Florian P Bayer, Atticus McCoy, Bernhard Kuster, Christopher M Rose. Defining the carrier proteome limit for single-cell proteomics. Nat Methods. 2021;18(1):76-83. https://doi.org/10.1038/s41592-020-01002-5
[145] Yang Liu, Shuang-Yan Ye, Shuai He, Dong-Mei Chi, Xiu-Zhi Wang, Yue-Feng Wen, et al. Single-cell and spatial transcriptome analyses reveal tertiary lymphoid structures linked to tumour progression and immunotherapy response in nasopharyngeal carcinoma. Nat Commun. 2024;15(1):7713. https://doi.org/10.1038/s41467-024-52153-4
[146] Samuel G Rodriques, Robert R Stickels, Aleksandrina Goeva, Carly A Martin, Evan Murray, Charles R Vanderburg, et al. Slide-seq: A scalable technology for measuring genome-wide expression at high spatial resolution. Science. 2019;363(6434):1463-1467. https://doi.org/10.1126/science.aaw1219
[147] Ke-Ran Li, Pei-Long Yu, Qi-Qi Zheng, Xin Wang, Xuan Fang, Lin-Chen Li, et al. Spatiotemporal and genetic cell lineage tracing of endodermal organogenesis at single-cell resolution. Cell. 2025;188(3):796-813.e24. https://doi.org/10.1016/j.cell.2024.12.012
[148] Amanda Janesick, Robert Shelansky, Andrew D Gottscho, Florian Wagner, Stephen R Williams, Morgane Rouault, et al. High resolution mapping of the tumor microenvironment using integrated single-cell, spatial and in situ analysis. Nat Commun. 2023;14(1):8353. https://doi.org/10.1038/s41467-023-43458-x
[149] Yufan Yang, Ziyuan Liu, Yerong Wei, Shuai He, Ancheng Gu, Zhiyong Li, et al. Single-cell multi-omics analysis reveals candidate therapeutic drugs and key transcription factor specifically for the mesenchymal subtype of glioblastoma. Cell Biosci. 2024;14(1):151. https://doi.org/10.1186/s13578-024-01332-3
[150] Fiorella C Grandi, Hailey Modi, Lucas Kampman, M Ryan Corces. Chromatin accessibility profiling by ATAC-seq. Nat Protoc. 2022;17(6):1518-1552. https://doi.org/10.1038/s41596-022-00692-9
[151] Jianli Lin, Xiaohui Xue, Yan Wang, Yuan Zhou, Jian Wu, Haoling Xie, et al. scNanoCOOL-seq: a long-read single-cell sequencing method for multi-omics profiling within individual cells. Cell Res. 2023;33(11):879-882. https://doi.org/10.1038/s41422-023-00873-5
[152] Andrew M Leader, John A Grout, Barbara B Maier, Barzin Y Nabet, Matthew D Park, Alexandra Tabachnikova, et al. Single-cell analysis of human non-small cell lung cancer lesions refines tumor classification and patient stratification. Cancer Cell. 2021;39(12):1594-1609.e12. https://doi.org/10.1016/j.ccell.2021.10.009
[153] Yuqiu Yang, Kaiwen Wang, Zeyu Lu, Tao Wang, Xinlei Wang. Cytomulate: accurate and efficient simulation of CyTOF data. Genome Biol. 2023;24(1):262. https://doi.org/10.1186/s13059-023-03099-1
[154] Luca Rappez, Mira Stadler, Sergio Triana, Rose Muthoni Gathungu, Katja Ovchinnikova, Prasad Phapale, et al. SpaceM reveals metabolic states of single cells. Nat Methods. 2021;18(7):799-805. https://doi.org/10.1038/s41592-021-01198-0
[155] Lianshun Xie, Hengxin Liu, Zhiwen You, Luyue Wang, Yiwen Li, Xinyue Zhang, et al. Comprehensive spatiotemporal mapping of single-cell lineages in developing mouse brain by CRISPR-based barcoding. Nat Methods. 2023;20(8):1244-1255. https://doi.org/10.1038/s41592-023-01947-3
[156] Irene Garcia-Gonzalez, Stefano Gambera, Susana F Rocha, Alvaro Regano, Lourdes Garcia-Ortega, Mariya Lytvyn, et al. iFlpMosaics enable the multispectral barcoding and high-throughput comparative analysis of mutant and wild-type cells. Nat Methods. 2025;22(2):323-334. https://doi.org/10.1038/s41592-024-02534-w
[157] Giuliana Monachino, Beatrice Zanchi, Luigi Fiorillo, Giulio Conte, Angelo Auricchio, Athina Tzovara, et al. Deep Generative Models: The winning key for large and easily accessible ECG datasets?. Comput Biol Med. 2023;167:107655. https://doi.org/10.1016/j.compbiomed.2023.107655
[158] Elena Denisenko, Belinda B Guo, Matthew Jones, Rui Hou, Leanne de Kock, Timo Lassmann, et al. Systematic assessment of tissue dissociation and storage biases in single-cell and single-nucleus RNA-seq workflows. Genome Biol. 2020;21(1):130. https://doi.org/10.1186/s13059-020-02048-6
[159] Mridusmita Saikia, Philip Burnham, Sara H Keshavjee, Michael F Z Wang, Michael Heyang, Pablo Moral-Lopez, et al. Simultaneous multiplexed amplicon sequencing and transcriptome profiling in single cells. Nat Methods. 2019;16(1):59-62. https://doi.org/10.1038/s41592-018-0259-9
[160] Abadi SAR, Mohammadi A, Koohi S. An automated ultra-fast, memory-efficient, and accurate method for viral genome classification. J Biomed Inform. 2023;139:104316. https://doi.org/10.1016/j.jbi.2023.104316
[161] Tera SP, Chinthaginjala R, Shahzadi I, Natha P, Rab SO. Deep learning approach for automated hMPV classification. Sci Rep. 2025;15(1):29068. Published 2025 Aug 8. https://doi.org/10.1038/s41598-025-14467-1
[162] Zhiwei Nie, Xudong Liu, Jie Chen, Zhennan Wang, Yutian Liu, Haorui Si, et al. A unified evolution-driven deep learning framework for virus variation driver prediction. Nat Mach Intell 7, 2025. 131–144 . https://doi.org/10.1038/s42256-024-00966-9
[163] Søren M Karst, Ryan M Ziels, Rasmus H Kirkegaard, Emil A Sørensen, Daniel McDonald, Qiyun Zhu, et al. High-accuracy long-read amplicon sequences using unique molecular identifiers with Nanopore or PacBio sequencing. Nat Methods. 2021;18(2):165-169. https://doi.org/10.1038/s41592-020-01041-y
[164] Junyue Cao, Malte Spielmann, Xiaojie Qiu, Xingfan Huang, Daniel M Ibrahim, Andrew J Hill, et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature. 2019;566(7745):496-502. https://doi.org/10.1038/s41586-019-0969-x
[165] Pierre Bost, Amir Giladi, Yang Liu, Yanis Bendjelal, Gang Xu, Eyal David, et al. Host-Viral Infection Maps Reveal Signatures of Severe COVID-19 Patients. Cell. 2020;181(7):1475-1488.e12.https://doi.org/10.1016/j.cell.2020.05.006
Type
Published
Data Availability Statement
None.
Issue
Section
License
Copyright (c) 2025 Life Conflux

This work is licensed under a Creative Commons Attribution 4.0 International License.







